Microchemistry /Electronmicoscropy
Microchemistry /Electronmicoscropy
The smallest clues
To understand more about the causes of eruptions, geologists have to look more closely into the fine details of the solidified magma samples to find a record of the conditions before and during eruption. Mineral crystals within magmas vary in composition depending on the surrounding magma and the temperature at which they are formed.
Why? do some volcanoes explode catatrophically with rapid, life threatening devastation? Recent research indicates that magma does not necessarily move directly from its source to an eruption. A magma chamber may contain stable reservoirs or layers of one composition with a lower temperature. Subsequent influx and mixing of a second higher temperature lava overheats the mixture, triggering an explosion. The 1991 Pinatubo eruption appears to have been triggered because hot, low-silica basalt magma penetrated a stable Reservoir of cooler, high-silica type, forming an explosive mixture. The explosion forced the closing of Clark Naval Air Station and interrupted numerous air flights because of ash clouds that damaged engines.
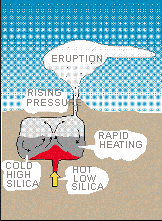
Mineral compositions from the 1991 eruption of Mt. Pinatubo indicate that low-silica magma at a temperature of about 1,250 C mixed with high-silica magma (780 C) just before the eruption.
Based on this information, volcanic rocks produced in previous eruptions were analysed. The results suggest that the 1991 eruption is the latest in a series of eruptions that were triggered by the mixing of magmas. Magma mixing has also triggered eruptions at a number of other volcanoes.
Shortly after World War II, physicists in the United States, England, Germany, and Japan began to perfect a new analytical instrument called the electron microscope. Instead of producing a visually magnified image, this new instrument accelerated and focused electrons through a column of magnetic lenses onto a small spot on the sample. The ability to magnify objects is limited by the energy or wavelength of the radiation that is used to observe the object. Because the accelerated electrons from the column have a much shorter wavelength than light, it is possible to produce images at much higher magnifications than can be obtained using an optical microscope. Today, the most powerful electron microscopes can produce images at magnifications as high as 1 million times.
When electrons are accelerated into an object, they interact with the atoms in that object and
produce three important types of radiation:
- X-rays (formed by bombarding a sample with electrons.)
- (2) the secondary electrons that are used to see the sample
- (3) back-scattered electrons, which are bounced back as a function of the mass of the sample.
In order to see objects smaller than what normal light allows, scientists have developed an instrument that accelerates electrons.
The Scanning Electron Microscope uses electromagnetic lenses to focus the electrons, since glass lenses cannot.
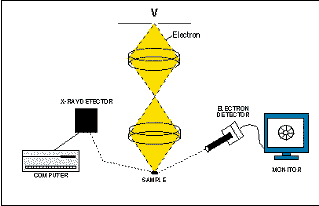
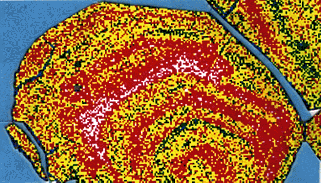
The secondary electrons are continuously detected, and the signal is directed to a television monitor where the image is displayed. Zooming in or backing out by changing the size of the raster area (hence changing the magnification), the scientist can use the enlarged image to aim the scanning electron microscope. At the same time, X-rays characteristic of the composition are generated. These X- rays can be detected by an X-ray analyser and used to create a map of the element's abundance.
In this example, calcium X-rays produced from a pinhead-size sample from the 1991 eruption of Mt. Pinatubo are mapped and colour coded by a scanning electron microscope to show the range of calcium content from high (white) to low (green).
Analysing a single particle of smoke
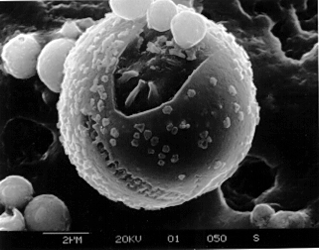
Because of their similarities, EMPs and SEMs overlap in their capabilities. The modern EMP has become a true hybrid that combines the viewing capability of the SEM with the analytical power of the electron microprobe. Both EMPs and SEMs are capable of obtaining images at magnifications over 100,000 times. These instruments can see and then analyse something that wouldn't show up with a light microscope, such as the following single particle of volcano smoke in this picture.
After seeing the invisible, the next question is "wonder what that's made of?" "Is it bad for our health?" Small samples like this particle of volcanic smoke, the size of a single human red blood cell, can be analysed by a scanning electron microscope in 4 minutes with errors of less than 1 percent.