Minerals
Minerals
back to Contents of Entire Course...
Definition of a Mineral
Atoms
Structure of Atoms
Crystal Structure
Ionic Substitution (Solid
Solution)
Composition of Minerals
Properties of Minerals
adapted to HTML from lecture notes of Prof. Stephen A. Nelson Tulane University
The Earth is composed of rocks. Rocks are aggregates of minerals. Minerals are composed of atoms. In order to understand rocks, we must first have an understanding of minerals. In order to understand minerals we must have some basic understanding of atoms - what they are and how they interact with one another to form minerals.
Definition of a Mineral:
Naturally formed; it forms in nature on its own (some say without the aid of humans)
- Generally a solid (though oil, gas...are also considered minerals in the economic sense)
- With a definite chemical composition (every time we see the same mineral it has the same chemical composition that can be expressed by a chemical formula).
- and a characteristic crystalline structure (atoms are arranged within the mineral in a specific ordered manner).
- Glass - can be naturally formed (volcanic glass called obsidian), is a solid, its chemical composition, however, is not always the same, and it does not have a crystalline structure. Thus, glass is not a mineral.
- Ice - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula H2O, and does have a definite crystalline structure when solid. Thus, ice is a mineral, but liquid water is not (since it is not solid).
- Halite (salt) - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula NaCl, and does have a definite crystalline structure. Thus halite is a mineral.
Atoms Atoms make up the chemical elements.
Each chemical element has nearly identical atoms. An atom is composed of three different particles:
- Protons -- positively charged, reside in the center of the atom called the nucleus
- Electrons -- negatively charged, orbit in a cloud around nucleus
- Neutrons -- no charge, reside in the nucleus.
Each element has the same number of protons and the same number of electrons. Number of protons = Number of electrons.
- Number of protons = atomic number.
- Number of protons + Number of neutrons = atomic weight.
Isotopes are atoms of the same element with differing numbers of neutrons. i.e. the number of neutrons may vary within atoms of the same element. Some isotopes are unstable which results in radioactivity. Example: K (potassium) has 19 protons. Every atom of K has 19 protons. Atomic number of K = 19. Some atoms of K have 20 neutrons, others have 21, and others have 22. Thus atomic weight of K can be 39, 40, or 41. 40K is radioactive and decays to 40Ar and 40Ca.
Structure of Atoms
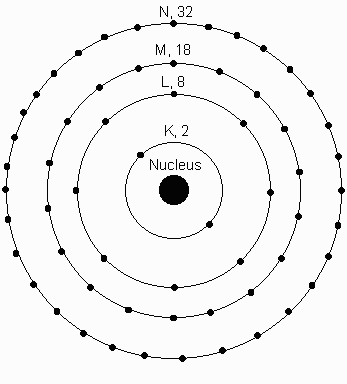
Electrons orbit around the nucleus in different shells, labeled from the innermost shell as K, L, M, N, etc. Each shell can have a certain number of electrons. The K-shell can have 2 Electrons, the L-shell, 8, the M-shell 18, N-shell 32. #electrons = 2N2 , where N=1 for the K shell, N=2 for the L shell, N=3 for the M shell, etc.
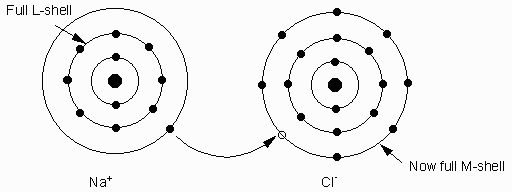
A stable electronic configuration for an atom is one with a completely filled outer shell. Thus, atoms often loose electrons or gain electrons to obtain stable configuration. Noble gases have completely filled outer shells, so they are stable. Examples He, Ne, Ar, Kr, Xe, Rn. Others like Na, K loose an electron. This causes the charge balance to become unequal. In fact to become + (positive) charged atoms called ions. Positively charged atoms = cations. Elements like F, Cl, O gain electrons to become ( - ) charged. ( -) charged ions are called anions.
The drive to attain a stable electronic configuration in the outermost shell along with the fact that this sometimes produces oppositely charged ions, results in the binding of atoms together.When atoms become attached to one another, we say that they are bonded together.
Types of bonding:
- Ionic bonding- caused by the force of attraction between ions of opposite charge. Example Na+1 and Cl-1. Bond to form NaCl (halite or salt).
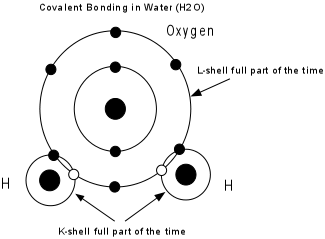
- Covalent bonding - Electrons are shared between two or more atoms so that each atom has a stable electronic configuration (completely filled outermost shell) part of the time.
- Metallic bonding -- Similar to covalent bonding, except innermost electrons are also shared. In materials that bond this way, electrons move freely from atom to atom and are constantly being shared. Materials bonded with metallic bonds are excellent conductors of electricity because the electrons can move freely through the material.
- Van der Waals bonding -- a weak type of bond that does not share or transfer electrons. Usually results in a zone along which the material breaks easily (cleavage). A good example is graphite
Several different bond types can be present in a mineral, and these determine the physical properties of the mineral.
Crystal Structure
Packing of atoms in a crystal structure requires an orderly and repeated atomic arrangement. Such an orderly arrangement needs to fill space efficiently and keep a charge balance. Since the size of atoms depends largely on the number of electrons, atoms of different elements have different sizes.
Example of NaCl :
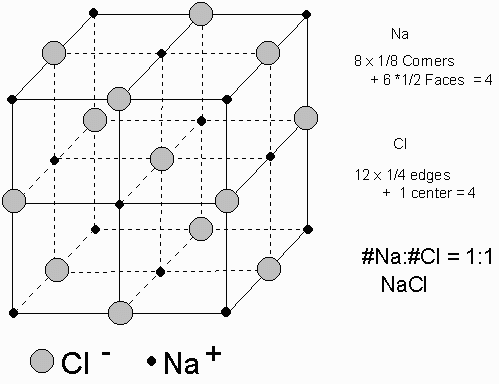
For each Na atom there is one Cl atom. Each Na is surrounded by Cl and each Cl is surrounded by Na. The charge on each Cl is -1 and the charge on each Na is +1 to give a charged balanced crystal.
The structure of minerals is often seen in the shape of crystals.
The law of constancy of interfacial angles --- Angles between the same faces on crystals of the same substance are equal. This is a reflection of ordered crystal structure
Crystal structure depends on the conditions under which the mineral forms.
Polymorphs are minerals with the same chemical composition but different crystal structures.
The conditions are such things as temperature (T) and pressure (P), because these effect ionic radii.
At high T atoms vibrate more, and thus distances between them get larger.
Crystal structure changes to accommodate the larger atoms.
At even higher T substances changes to liquid and eventually to gas.
Liquids and gases do not have an ordered crystal structure and are not minerals.
Increase in P pushes atoms closer together.
This makes for a more densely packed crystal structure.
Examples:
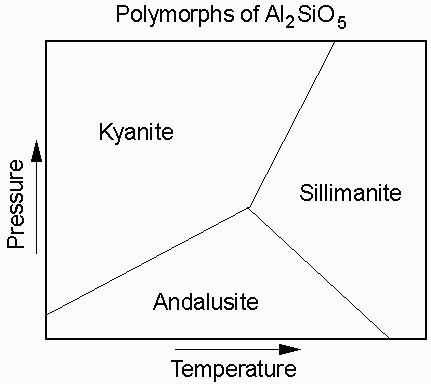
The compound Al2SiO5 has three different polymorphs that depend on the temperature and pressure at which the mineral forms. At high P the stable form of Al2SiO5 is kyanite, at low P the stable from is andalusite, and at high T it is sillimanite.
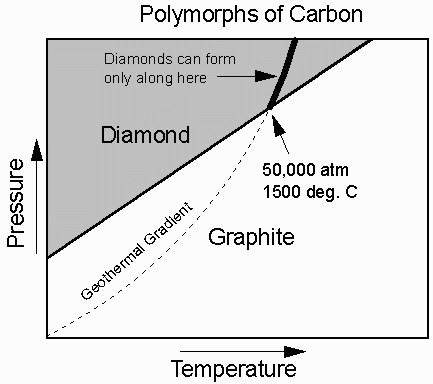
Carbon (C) has two different polymorphs.
At low T and P pure carbon is the mineral graphite, (pencil lead), a very soft mineral.
At higher T and P the stable form is diamond, the hardest natural substance known.
In the diagram, the geothermal gradient ( how temperature varies with depth or pressure in the Earth) is superimposed on the stability fields of Carbon. Thus we know that when we find diamond it came from someplace in the Earth where the temperature is greater than 1500oC and the pressure is higher than 50,000 atmospheres (equivalent to a depth of about 170 km).
CaCO3 - Low Pressure form is Calcite, High Pressure form is Aragonite
Ionic Substitution (Solid Solution)
Ionic substitution - (also called solid solution), occurs because some elements (ions) have the same size and charge, and can thus substitute for one another in a crystal structure.
Examples:
- Olivines Fe2SiO4 and Mg2SiO4. Fe+2 and Mg+2 are about the same size, thus they can substitute for one another in the crystal structure and olivine thus can have a range of compositions expressed as the formula (Mg,Fe)2SiO4.
- Alkali Feldspars: KAlSi3O8 (orthoclase) and NaAlSi3O8, (albite)K+1 can substitute for Na+1
- Plagioclase Feldspars: NaAlSi3O8 (albite) and CaAl2Si2O8 (anorthite) NaSi+5 can substitutes for CaAl+5 (a complex solid solution).
Composition of Minerals
The variety of minerals we see depend on the chemical elements available to form them. In the Earth's crust the most abundant elements are as follows:
- O, Oxygen 45.2% by weight
- Si, Silicon 27.2%
- Al, Aluminum 8.0%
- Fe, Iron 5.8%
- Ca, Calcium 5.1%
- Mg, Magnesium 2.8%
- Na, Sodium 2.3%
- K, Potassium 1.7%
- Ti ,Titanium 0.9%
- H, Hydrogen 0.14%
- Mn, Manganese 0.1%
- P, Phosphorous 0.1%
Note that Carbon (one of the most abundant elements in life) is not among the top 12.
Because of the limited number of elements present in the Earth's crust there are only about 3000 minerals known. Only 20 to 30 of these minerals are common. The most common minerals are those based on Si and O: the Silicates. Silicates are based on SiO4 tetrahedron. 4 Oxygens covalently bonded to one silicon atom.
Properties of Minerals
Physical properties of minerals allow us to distinguish between minerals and thus identify them, as you will learn in lab. Among the common properties used are:
- Habit - shape
- Color
- Streak and (color of fine powder of the mineral)
- Luster -- metallic, vitreous, pearly, resinous (reflection of light)
- Cleavage (planes along which the mineral breaks easily)
- Density (mass/volume)
- Hardness: based on Mohs hardness scale as follows:
- Talc
- gypsum (fingernail)
- calcite (penny)
- fluorite
- apatite (knife blade)
- orthoclase (glass)
- quartz
- topaz
- corundum
- Diamond

