Microfossils / Palynology / Radiolarians
Microfossils / Palynology / Radiolarians
from the Postgraduate Unit of Micropalaeontology at University College London
see their excellent site if you want more than just the thumbnails!
Calcareous Nannofossils
Conodonts
Diatoms
Foraminifera
Ostracods
Palynology
Radiolaria
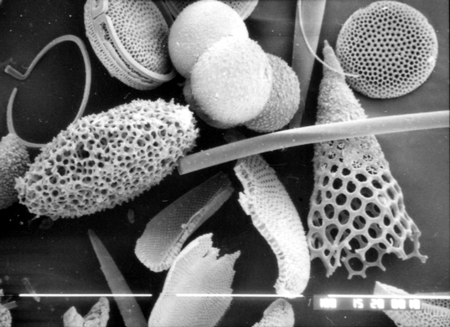
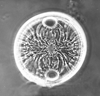
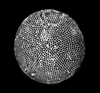
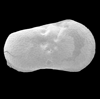
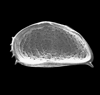
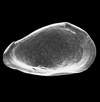
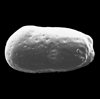
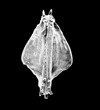
The picture above is a scanning electron microscope (SEM)
image of a selection of microfossils.
The scale bars are 100 microns (one tenth of a millimetre)
Calcareous Nannofossils
Calcareous nannofossils include the coccoliths and coccospheres of haptophyte algae and the associated nannoliths which are of unknown provenance. The organism which creates the coccosphere is called a coccolithophore, they are phytoplankton (autotrophs that contain chloroplasts and photosynthesise). Their calcareous skeletons are found in marine deposits often in vast numbers, sometimes making up the major component of a particular rock, such as the chalk of England. One freshwater species has been reported. Formally coccolithophores are separated from other phytoplankton such as diatoms by the presence of a third flagella-like appendage called a haptonema, although the flagella bearing stage is often only one of a multi-stage life cycle.A coccolith is a single disc-like plate which is secreted by the algal organism and held in combination with several other, sometimes varying shaped plates by an organic coating to form the coccosphere. On death the individual coccoliths invariably become separated and it is these that are most commonly preserved in the sedimentary record. Occasionally complete coccospheres are preserved and provide valuable information, particularly regarding coccospheres which possess two or more morphologicaly different coccoliths. There are two forms of coccoliths, the holococcoliths which are formed from calcite crystals which are essentially identical in shape and size and the heterococcoliths which are formed from larger calcite crystals which vary in size and shape. Most living forms are known to produce only heterococcoliths and then only during the non-motile stage of their life cycle. Those that do produce holococcoliths do so only during their motile stage.
The first recorded use of the term "coccoliths" is from Ehrenberg's 1836 study of the chalk from the island of Rugen in the Baltic Sea. Ehrenberg and other early workers beleived coccoliths to have an inorganic origin. It was not untill the second half of the nineteenth century when Wallich found coccoliths joined to form coccospheres that an organic origin was suggested. Even after the publication of Sorby's 1861 paper, following which the organic origin of coccoliths was generally accepted, Ehrenberg remained unconvinced. The 1872 HMS Challenger expedition recovered coccospheres from the upper water layers and correctly concluded that they were the skeletons of calcareous algae. The term nannoplankton was coined by Lohmann in 1902. The study of coccolithophores has flourished since the 1960's, with much ground breaking work done on their biology as well as on the systematics of fossil and living forms. The Deep Sea Drilling Project (DSDP), now the Ocean Drilling Program (ODP), brought the stratigraphic value of calcareous nannofossils to the attention of industry as well as the scientific community. Today, due to the speed of preparation, calcareous nannofossils have bec ome the preferred tool for quick accurate stratigraphic age determination in post-Palaeozoic calcareous sequences.
First recorded occurrences of calcareous nannofossils (nannoliths) are from the late Triassic (Carnian). The locations from which the earliest nannofossils are found include; the Northern and Southern Calcareous Alps, Timor, North-West Australia and Queen Charlotte Islands (Canada), all low latitude sites at the time. There are many claims for earlier occurrences but a lack of substantiated evidence means these must be excluded. One consequence of the first occurrence of calcareous nannofossils in the late Triassic lies in the fact that this was the first time open ocean planktonic organisms utilised calcareous skeletons and exported calcium carbonate into the deep oceans. This has important repercussions in terms of biogeochemical cycles. Today coccolithophores are one of the most important forms of phytoplankton found in the oceans, and may be described as the grass of the sea.
The classification of calcareous nannoplankton is carried out under the International Code of Botanical Nomenclature. They are formally classified in the Kingdom Protoctista, Phylum (or Division) Haptophyta, Class Prymnesiophyceae. Classification is complicated by the fact that some species are dimorphic, that is they possess more than one coccolith on a single coccosphere. This may lead to the belief that two species exist where in fact there is only one. Also, pleomorphism (where a holococcolith phase alternates with a heterococcolith phase) may also result in coccoliths being placed in different species or even genera when in fact they are simply different stages in the life cycle of the same species.
As the groups name suggests calcareous nannofossils are small, generally less than 30 microns across and usually between 5 and 10 microns (individual coccoliths). This has advantages and disadvantages. Advantages include:
- Good preservation, their small size makes mechanical damage unlikely.
- Widespread distribution, as part of the phytoplankton coccolithophores are distributed throughout the photic zone (predominantly the upper 50m of the water column) across almost all marine habitats.
- A very large number of individual coccoliths may be preserved in a tiny amount of sediment hence only very small quantities of sample are needed to produce statistically valid results.
Disadvantages include:
- Because of dissolution of calcium carbonate at depth in sea water (called the carbonate compensation depth (CCD)), preservation is compromised in deep water sediments.
- Because of their small size and resistance to mechanical breakdown nannofossils can be reworked, great care is therefore needed especially when utilising nannofossils for biostratigraphic studies.
- Again, because of the small size the opportunities for contamination are high, although careful and thorough preparation and collection techniques should significantly reduce this risk.
The defining feature of the haptophytes is the flagella-like haptonema which is generally coiled. It differs from the flagella proper in its internal structure and its basal attachment. During the non-motile phase the flagella disappear but the haptonema often remains, the exact function of the haptonema is not fully understood. The algal cell contains a nucleus and two golden-brown chloroplasts which may be moved around the cell to optimise collection of available light. The cell also contains mitochondria which contain enzymes which produce the energy for cell function, vacuoles which deal with waste products and the Golgi body which is the site of coccolith secretion in many species. In many species overlapping oval organic scales coat the outer cell membrane. These have concentric ridges on their distal faces and radiating ridges on their proximal faces. It seems the organic scales act as bases for the precipitation of the calcite coccoliths. A variety of coccolith secretion strategies have been observed in different species, however it is probably true of all coccolithophores that the production of coccoliths is controlled by light. Emiliania huxleyi has been observed to start coccolith production within half an hour of being introduced to light, and produce an individual coccolith in one hour and a complete coccosphere in about thirty hours.
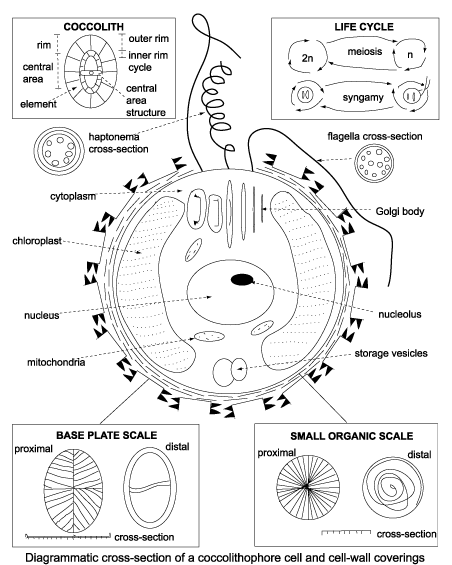
Above diagram from Bown,P.(Ed.), 1998, Calcareous Nannofossil Biostratigraphy. Chapman and Hall.
The function of coccoliths is not known but may be one or more of four basic possibilities:
- Protection; from bacteria, physical damage, predators such as copepods or to form a chemical buffer zone.
- Flotation and buoyancy; aspherical forms may reduce sinking rates, the loss or addition of coccoliths may be a strategy employed to regulate position in the water column in order to optimise light or nutrient availability.
- Light regulation; coccoliths may reflect sunlight protecting the cell from high light levels in the upper water column or refract sunlight into the cell allowing life in the lower photic zone.
- Biochemistry; the cell may secrete calcite in order to expell a metabolic by-product enhancing the bichemical efficiency of the cell.
Please remember all preparation techniques require the use of hazardous materials and equipment and should only be carried out in properly equiped laboratories, wearing the correct safety clothing and under the supervision of qualified staff.
Smear slides are produced by first cleaning a hand specimen by paring the outer surfaces off. A fine "dust" of material is then scraped off onto a cover slip. This is then moistened with distilled water and spread across the cover slip with a suitable utensile such as a wooden tooth pick. This takes a certain amount of experience to get right but when the corrrect coverage is obtained the cover slip is placed on a hot plate to dry. Once dry the cover slip is inverted and glued to a slide using Norland optical adhesive which is cured under U.V. light. Centrifuge slides are produced by first cleaning the sample as in the smear slide technique and then scraping a dust of material into a centrifuge tube. This is topped up with distilled water and spun at 350 rpm in a centrifuge for about two minutes. The pellet is then put to one side and the supernatant kept. The supernatant is then re-suspended and centrifuged at 1000rpm for four minutes this time keeping the pellet. This re-suspending and centrifuging at 1000rpm may need to be repeated several times depending on the lithology of the sample. After centrifuging the sample is dilluted to a slightly milky consistency with distilled water and strewn on to a cover slip placed on a hot plate and left to dry. The cover slip can then be mounted as in the smear slide technique. One of the major advantages calcareous nannofossils have over other microfossil groups, particularly in terms of industrial application is the speed at which samples can be prepared. Simple smear slides can be made in minutes and even centrifuge preparations are ready in less than half an hour. Another advantage is that no harmful or dangerous chemicals are needed nor even a fume cupboard. This makes calcareous nannofossils an extremely useful and widely used biostratigraphic tool especially on offshore drilling platforms and ships.
Since individualcoccoliths preserve fine structural crystallographic detail in calcite observation techniques depend on the use of petrological microscopes. The calcite crystals forming hetero- and holococcoliths often have differently oriented optic axes which produce distinctive extinction patterns under crossed nicols of a polarising microscope. Transmitted, cross polarised light is regularly used but phase contrast and bright field settings may also be advantageous. Scanning Electron Microscopy has become more widely available and greatly enhanced the study of nannofossils. Much of the work on the fine structure and formation of coccoliths has been made possible by scanning electron microscopes.
The following images are of a representative selection of calcareous nannofossils aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and if possible a species name followed by its age range, the site location from which the sample was obtained and the magnification at which the image was taken or its size in microns. PC (Phase Contrast), XPL (Crossed Polarised Light) SEM (Scanning Electron Microscope). Typical and selected marker species are illustrated from each main period of the geological column in which calcareous nannofossils occur.
Triassic and Jurassic |

|
| Anulasphaera helvetica Grun and Zweili, 1980 |
| Callovian (Middle Jurassic) |
| Denver Borehole, UK |
| PC side view |
 |
| Anulasphaera helvetica Grun and Zweili, 1980 |
| Callovian (Middle Jurassic) |
| Denver Borehole, UK |
| XPL side view |
 |
| Anulasphaera helvetica Grun and Zweili, 1980 |
| Callovian (Middle Jurassic) |
| Denver Borehole, UK |
| (SEM) distal view |
 |
| Anulasphaera helvetica Grun and Zweili, 1980 |
| Callovian (Middle Jurassic) |
| Denver Borehole, UK |
| (SEM) proximal oblique view |
 |
| Stephanolithion bigotii bigotii Deflandre, 1939 |
| Lower Oxfordian (Upper Jurassic) |
| Cleveland Farm Pit, wiltshire, UK |
| XPL |
 |
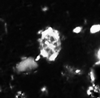
| Stephanolithion bigotii bigotii Deflandre, 1939 |
| Upper Kimmeridgian (Upper Jurassic) |
| Gorodische, Russia |
| SEM |
 |
| Stephanolithion speciosum octum Deflandre in Deflandre and Fert, 1954 ssp. Rood and Barnard, 1972 |
| Lower Bathonian (Middle Jurassic) |
| Port en Bessin, N. France |
| XPL |
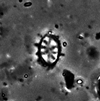 |
| Stephanolithion speciosum octum Deflandre in Deflandre and Fert, 1954 ssp. Rood and Barnard, 1972 |
| Lower Bathonian (Middle Jurassic) |
| Port en Bessin, N. France |
| PC |
 |
| Stephanolithion speciosum Deflandre in Deflandre and Fert, 1954 ssp. octum Rood and Barnard, 1972 |
| Upper Bajocian-Lower Callovian (Middle Jurassic) |
| Escoville, France |
| distal view |
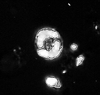 |
| Biscutum novum (Goy,1979) Bown, 1987 |
| Aalenian/Bajocian |
| Brenha, Portugal |
| XPL |
 |
| Biscutum novum (Goy,1979) Bown, 1987 |
| Lower Toarcian |
| Trimeusel, Germany |
| distal view |
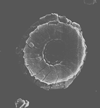 |
| Biscutum novum (Goy,1979) Bown, 1987 |
| Upper Toarcian |
| Ballrechten, Germany |
| proximal view |
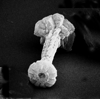 |
| Carinolithus superbus (Deflandre in Deflandre and Fert, 1954) Prins in Grun et al, 1974 |
| Lower Toarcian-Lower Bajocian |
| Ilminster, UK |
| proximal oblique view SEM |
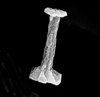 |
| Carinolithus superbus (Deflandre in Deflandre and Fert, 1954) Prins in Grun et al, 1974 |
| Lower Toarcian-Lower Bajocian |
| Ilminster, UK |
| side view SEM |
 |
| Crucirhabdus minutus Jafar, 1983 |
| Norian-Rhaetian (Upper Triassic) |
| Fischerwiese, Austria |
| XPL distal view |
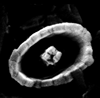 |
| Crucirhabdus minutus Jafar, 1983 |
| Norian-Rhaetian (Upper Triassic) |
| Weissloferbach, S. Germany |
| distal view SEM |
 |
| Lotharingius haufii Grun and Zweili in Grun et al, 1974 |
| Lower Toarcian |
| Untersturmig, Germany |
| XPL |
 |
| Lotharingius haufii Grun and Zweili in Grun et al, 1974 |
| Upper Pliensbachian-Upper Bathonian |
| Untersturmig, Germany |
| PC |
 |
| Lotharingius haufii Grun and Zweili in Grun et al, 1974 |
| Upper Pliensbachian-Upper Bathonian |
| Untersturmig, Germany |
| SEM (collapsed coccosphere) |
 |
| Parhabdolithus liasicus distinctus Deflandre in Grasse, 1952 ssp. Bown, 1987 |
| Hettangian-Lower Toarcian |
| Timor |
| XPL plan view |
 |
| Parhabdolithus liasicus distinctus Deflandre in Grasse, 1952 ssp. Bown, 1987 |
| Hettangian-Lower Toarcian |
| Timor |
| XPL side view |
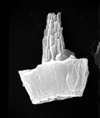 |
| Parhabdolithus liasicus distinctus Deflandre in Grasse, 1952 ssp. Bown, 1987 |
| Hettangian-Lower Toarcian |
| Mochras Borehole, UK |
| side view (SEM) |
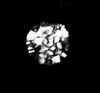 |
| Prinsiosphaera triassica Jafar, 1983 |
| Norian-Rhaetian |
| Weissloferbach S. Germany |
| XPL |
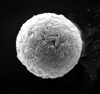 |
| Prinsiosphaera triassica Jafar, 1983 |
| Norian-Rhaetian |
| ODP Site 761, Wombat Plateau, NW Australian shelf |
| SEM |
| TOP |
Lower Cretaceous |
 |
| Axopodorhabdus albianus (Black, 1967) Wind and Wise in Wise and Wind, 1977 |
| Middle Albian-Upper Cenomanian |
| Folkestone, UK |
| XPL |
 |
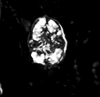
| Axopodorhabdus albianus (Black, 1967) Wind and Wise in Wise and Wind, 1977 |
| Middle Albian-Upper Cenomanian |
| English Channel Borehole R330, UK |
| distal view SEM |
 |
| Calcicalathina oblongata (Worsley, 1971) Thierstein, 1971 |
| Lower Valanginian-Lower Barremian |
| Bulgaria |
| XPL distal view |
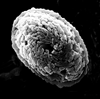 |
| Calcicalathina oblongata (Worsley, 1971) Thierstein, 1971 |
| Lower Valanginian-Lower Barremian |
| DSDP Site 547B, Atlantic Ocean |
| distal view SEM |
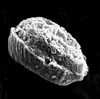 |
| Calcicalathina oblongata (Worsley, 1971) Thierstein, 1971 |
| Lower Valanginian-Lower Barremian |
| DSDP Site 547B, Atlantic Ocean |
| side view SEM |
 |
| Ceratolithina bicornuta Perch-Nielsen, 1988 |
| Middle Albian-Upper Albian |
| Folkestone, UK |
| XPL |
 |
| Corollithion kennedyi |
| Cenomanian |
| Lydden Spout, Folkestone, UK |
| XPL |
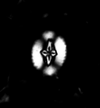 |
| Cruciellipsis cuvillieri (Manivit, 1966) Thierstein, 1971 |
| Lower Berriasian-Upper Hauterivian |
| DSDP Site 397, E.Atlantic Ocean |
| XPL |
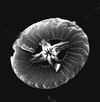 |
| Cruciellipsis cuvillieri (Manivit, 1966) Thierstein, 1971 |
| Lower Berriasian-Upper Hauterivian |
| DSDP Site 547B, Atlantic Ocean |
| distal view SEM |
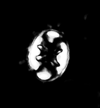 |
| Eiffellithus turriseiffelii (Deflandre in Deflandre and Fert, 1954) Reinhardt, 1965 |
| Upper ALbian-Upper Maastrichtian |
| Folkestone, UK |
| XPL distal view |
 |
| Eprolithus floralis (Stradner, 1962) Stover, 1966 |
| Lower Aptian-?Lower Campanian |
| Folkestone, UK |
| XPL distal view |
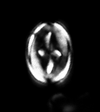 |
| Gartnerago segmentatum |
| Cenomanian-Maastrichtian |
| Langdon Stairs, Dover, Kent, UK |
| XPL distal view |
 |
| Micrantholithus obtusus Stradner, 1963 |
| Berriasian-Upper Aptian |
| Speeton, UK |
| XPL |
 |
| Micrantholithus obtusus Stradner, 1963 |
| Berriasian-Upper Aptian |
| DSDP Site 398D, Atlantic Ocean |
| SEM |
 |
| Nannoconus abundans Stradner and Grun, 1973 |
| Barremian-?Lower Aptian |
| Speeton, UK |
| XPL |
 |
| Nannoconus abundans Stradner and Grun, 1973 |
| Barremian-?Lower Aptian |
| Speeton, UK |
| side view SEM |
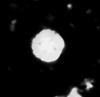 |
| Prediscosphaera columnata (Stover, 1966) Perch-Nielsen, 1984 |
| Lower Albian-Turonian |
| Folkestone, UK |
| XPL distal view |
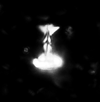 |
| Prediscosphaera columnata (Stover, 1966) Perch-Nielsen, 1984 |
| Lower Albian-Turonian |
| Folkestone, UK |
| XPL side view |
 |
| Prediscosphaera columnata (Stover, 1966) Perch-Nielsen, 1984 |
| Lower Albian-Turonian |
| Copt Point, UK |
| side view SEM |
 |
| Watznaueria barnesae (Black in Black and Barnes, 1959) Perch-Nielsen, 1968 |
| Lower Bajocian-Maastrichtian |
| Gorodische, Russia |
| XPL |
 |
| Watznaueria barnesae (Black in Black and Barnes, 1959) Perch-Nielsen, 1968 |
| Lower Bajocian-Maastrichtian |
| Speeton, UK |
| SEM (Coccosphere) |
 |
| Watznaueria britannica (Stradner, 1963) Reinhardt, 1964 |
| Lower Bajocian-Lower Cenomanian |
| Cleveland Farm Pit, Wiltshire, UK |
| XPL |
 |
| Zeugrhabdotus erectus (Deflandre in Deflandre and Fert, 1954) Reinhardt, 1965 |
| Lower Pliensbachian?-Upper Maastrichtian |
| Cleveland Farm Pit, Wiltshire, UK |
| XPL |
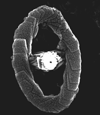 |
| Zeugrhabdotus erectus (Deflandre in Deflandre and Fert, 1954) Reinhardt, 1965 |
| Lower Pliensbachian?-Upper Maastrichtian |
| Mochras Borehole, UK |
| distal view SEM |
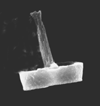 |
| Zeugrhabdotus erectus (Deflandre in Deflandre and Fert, 1954) Reinhardt, 1965 |
| Lower Pliensbachian?-Upper Maastrichtian |
| Dorset, UK |
| side view SEM |
| TOP |
Upper Cretaceous |
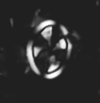 |
| Arkhangelskiella cymbiformis Vekshina, 1959 |
| Campanian-Maastrichtian |
| DSDP Site 249, Indian Ocean |
| XPL distal view |
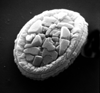 |
| Arkhangelskiella cymbiformis Vekshina, 1959 |
| Campanian-Maastrichtian |
| Keswick, Norfolk, UK |
| oblique distal view SEM |
 |
| Eiffellithus eximius (Stover, 1966) Perch-Nielsen, 1968 |
| Turonian-Campanian |
| Zoe C BH, South Africa |
| XPL |
 |
| Eiffellithus eximius (Stover, 1966) Perch-Nielsen, 1968 |
| Turonian-Campanian |
| Zoe C BH, South Africa |
| XPL rotated |
 |
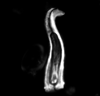
| Lithastrinus grillii Stradner, 1962 |
| Coniacian-Campanian |
| near Plymouth Bluff, Lowndes County, Mississippi, USA |
| XPL |
 |
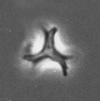
| Lucianorhabdus cayeauxii Deflandre, 1959 |
| Coniacian-Maastrichtian |
| near Portland, Dallas County, Alabama, USA |
| XPL |
 |
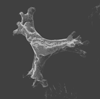
| Marthasterites furcatus (Deflandre in Deflandre and Firt, 1954) Deflandre, 1959 |
| Turonian-Campanian |
| DSDP Site 258, E.Indian Ocean |
| XPL |
 |
| Marthasterites furcatus (Deflandre in Deflandre and Firt, 1954) Deflandre, 1959 |
| Turonian-Campanian |
| DSDP Hole 550B, NE Atalntic Ocean |
| SEM |
 |
| Microrhabdulus decoratus Deflandre, 1959 |
| Cenomanian-Maastrichtian |
| DSDP Site 401, NE Atlantic Ocean |
| SEM |
 |
| Microrhabdulus decoratus Deflandre, 1959 |
| Cenomanian-Maastrichtian |
| DSDP Site 249, W. Indian Ocean |
| XPL |
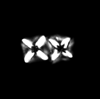 |
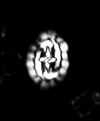
| Micula staurophora (Gardet, 1955) Stradner, 1963 |
| Coniacian-Maastrichtian |
| near Ripley, Tippah County, Mississippi, USA |
| XPL |
 |
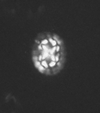
| Prediscosphaera arkhangelskyi (Reinhardt, 1965) Perch-Nielsen, 1984 |
| Santonian-Maastrichtian |
| DSDP Site 249, W. Indian Ocean |
| XPL |
 |
| Prediscosphaera cretacea (Arkhangelsky, 1912) Gartner, 1968 |
| Cenomanian-Maastrichtian |
| DSDP Site 249, W. Indian Ocean |
| XPL |
 |
| Prediscosphaera cretacea (Arkhangelsky, 1912) Gartner, 1968 |
| Cenomanian-Maastrichtian |
| near Plymouth Bluff, Lowndes County, Alabama, USA |
| XPL |
 |
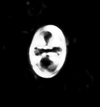
| Quadrum gartneri Prins and Perch-Nielsen in Manivit et al, 1977 |
| Turonian-?Maastrichtian |
| DSDP Site 217, N. Indian Ocean |
| XPL |
 |
| Tranolithus orionatus (Reinhardt, 1966a) Reinhardt, 1966b |
| Albian-Maastrichtian |
| Folkestone, UK |
| XPL |
 |
| Uniplanarius trifidus (Stradner in Stradner and Papp, 1961) Hattner and Wise, 1980 |
| Campanian-Maastrichtian |
| DSDP Site 217 N. Indian Ocean |
| XPL |
 |
| Uniplanarius trifidus (Stradner in Stradner and Papp, 1961) Hattner and Wise, 1980 |
| Campanian-Maastrichtian |
| DSDP Site 241 W. Indian Ocean |
| XPL |
| TOP |
Palaeogene |
 |
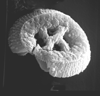
| Chiasmolithus solitus (Bramlette and Sullivan, 1961) Locker, 1968 |
| Lutetian-Bartonian (Middle Eocene) |
| Whitecliff Bay, UK |
| PC |
 |
| Chiasmolithus solitus (Bramlette and Sullivan, 1961) Locker, 1968 |
| Lutetian-Bartonian (Middle Eocene) |
| Fayum, Egypt |
| oblique distal view SEM |
 |
| Discoaster tanii Bramlette and Riedel, 1954 |
| Middle Eocene-Oligocene |
| Hampden Beach, New Zealand |
| distal view SEM |
 |
| Discoaster saipanensis Bramlette and Riedel, 1954 |
| Middle-Upper Eocene |
| Fayum, Egypt |
| distal view SEM |
 |
| Discoaster saipanensis Bramlette and Riedel, 1954 |
| Middle-Upper Eocene |
| Benidorm, Spain |
| distal view SEM |
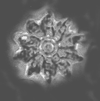 |
| Discoaster saipanensis Bramlette and Riedel, 1954 |
| Middle-Upper Eocene |
| Whitecliff Bay, UK |
| PC |
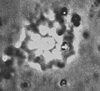 |
| Discoaster kuepperi Sradner, 1959 |
| Lower-Middle Eocene |
| North Sea, UK |
| PC |
 |
| Discoaster kuepperi Sradner, 1959 |
| Lower-Middle Eocene |
| Benidorm, Spain |
| proximal view SEM |
 |
| Discoaster kuepperi Sradner, 1959 |
| Lower-Middle Eocene |
| Benidorm, Spain |
| distal view SEM |
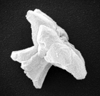 |
| Discoaster kuepperi Sradner, 1959 |
| Lower-Middle Eocene |
| Benidorm, Spain |
| side view SEM |
 |
| Cruciplacolithus primus Perch-Nielsen, 1977 |
| Upper Palaeocene |
| St. Paul Monastery, Egypt |
| oblique distal view SEM |
 |
| Neococcolithus dubius (Deflandre in Deflandre and Fert, 1954) Black, 1967 |
| Lower-Upper Eocene |
| Whitecliff Bay, UK |
| oblique distal view SEM |
 |
| Neococcolithus dubius (Deflandre in Deflandre and Fert, 1954) Black, 1967 |
| Lower-Upper Eocene |
| Whitecliff Bay, UK |
| distal view SEM |
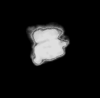 |
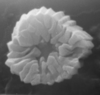
| Fasciculithus tympaniformis Hay and Mohler in Hay et al, 1967 |
| Upper Palaeocene-Lower Eocene |
| Pegwell Bay, Kent, UK |
| XPL |
 |
| Fasciculithus tympaniformis Hay and Mohler in Hay et al, 1967 |
| Upper Palaeocene-Lower Eocene |
| St. Paul Monastery, Egypt |
| oblique proximal view |
 |
| Fasciculithus tympaniformis Hay and Mohler in Hay et al, 1967 |
| Upper Palaeocene-Lower Eocene |
| St. Paul Monastery, Egypt |
| proximal view |
 |
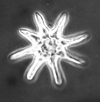
| Sphenolithus moriformis (Bronniman and Stradner, 1960) Brmlette and Wilcoxon, 1967 |
| Palaeocene-Pliocene |
| DSDP Site 590B, S.W Pacific |
| SEM |
 |
| Sphenolithus moriformis (Bronniman and Stradner, 1960) Brmlette and Wilcoxon, 1967 |
| Palaeocene-Pliocene |
| DSDP Site 593, S.W Pacific |
| SEM |
 |
| Discoaster lodoensis Bramlette and Riedel, 1954 |
| Palaeocene-Pliocene |
| Benidorm, Spain |
| XPL |
 |
| Discoaster lodoensis Bramlette and Riedel, 1954 |
| Palaeocene-Pliocene |
| Benidorm, Spain |
| proximal view SEM |
 |
| Discoaster lodoensis Bramlette and Riedel, 1954 |
| Palaeocene-Pliocene |
| Benidorm, Spain |
| distal view SEM |
| TOP |
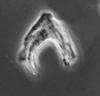
Neogene |
 |
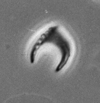
| Amaurolithus amplificus (Bukry and Percival) Gartner and Bukry, 1975 |
| Upper Miocene-Pliocene |
| Manavgat, S.Turkey |
| XPL |
 |
| Amaurolithus amplificus (Bukry and Percival) Gartner and Bukry, 1975 |
| Upper Miocene-Pliocene |
| Manavgat, S.Turkey |
| PC |
 |
| Coccolithus pelagicus (Wallich, 1871) Schiller, 1930 |
| Lower Palaeocene-Recent |
| N. Atlantic off S.W coast of Iceland |
| SEM entire coccosphere |
 |
| Coccolithus pelagicus (Wallich, 1871) Schiller, 1930 |
| Lower Palaeocene-Recent |
| ODP Site 1052b, Western N. Atlantic |
| SEM distal view 10 microns |
 |
| Coccolithus pelagicus (Wallich, 1871) Schiller, 1930 |
| Lower Palaeocene-Recent |
| ODP Site 1052b, Western N. Atlantic |
| SEM proximal view 10 microns |
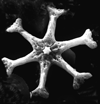 |
| Discoaster challengeri Bramlette and Riedel, 1954 |
| Miocene-Pliocene |
| Ghajn Tuffieha Bay, Malta |
| SEM proximal view |
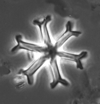 |
| Discoaster challengeri Bramlette and Riedel, 1954 |
| Miocene-Pliocene |
| G. Mihmandar Borehole, Malta |
| PC |
 |
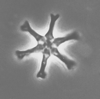
| Discoaster exilis Martini and Bramlette, 1963 |
| Middle Miocene |
| Ghajn Tuffieha Bay, Malta |
| proximal view SEM |
 |
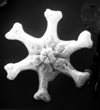
| Discoaster exilis Martini and Bramlette, 1963 |
| Middle Miocene |
| Ghajn Tuffieha Bay, Malta |
| PC |
 |
| Discoaster variabilis Martini and Bramlette, 1963 |
| Middle Miocene-Pliocene |
| Ghajn Tuffieha Bay, Malta |
| proximal view SEM |
 |
| Discoaster variabilis Martini and Bramlette, 1963 |
| Middle Miocene-Pliocene |
| Ghajn Tuffieha Bay, Malta |
| PC |
 |
| Florisphaera profunda Okado and Honjo, 1973 |
| Middle Miocene-Recent |
| Almerian Canyon, Western Mediterranian Sea |
| 10 microns SEM |
 |
| Gephyrocapsa oceanica Kamptner, 1943 |
| Pleistocene-Recent |
| Almerian Canyon, Western Mediterranian Sea |
| 10 microns SEM |
 |
| Calcidiscus tropicus Kamptner, 1956 |
| Lower Miocene-Recent |
| DSDP Site 593, S.W Pacific Ocean |
| SEM |
 |
| Calcidiscus tropicus Kamptner, 1956 |
| Lower Miocene-Recent |
| DSDP Site 593, S.W Pacific Ocean |
| SEM |
 |
| Helicosphaera carteri (Wallich, 1877) Kamptner, 1954 |
| Upper Oligocene-Recent |
| DSDP Site 590B, S.W Pacific Ocean |
| XPL |
 |
| Helicosphaera carteri (Wallich, 1877) Kamptner, 1954 |
| Upper Oligocene-Recent |
| DSDP Site 590B, S.W Pacific Ocean |
| proximal view SEM |
 |
| Reticulofenestra pseudoumbilica (Gartner) Gartner, 1969 |
| Miocene-Pliocene |
| Ghajn Tuffieha Bay, Malta |
| proximal view SEM |
 |
| Reticulofenestra pseudoumbilica (Gartner) Gartner, 1969 |
| Miocene-Pliocene |
| Ghajn Tuffieha Bay, Malta |
| coccosphere SEM |
 |
| Sphenolithus heteromorphus Deflandre, 1953 |
| Lower Miocene-Middle Miocene |
| DSDP Site 593, S.W Pacific Ocean |
| SEM |
 |
| Sphenolithus heteromorphus Deflandre, 1953 |
| Lower Miocene-Middle Miocene |
| DSDP Site 590B, S.W Pacific Ocean |
| SEM |
| TOP |
Conodonts
Conodont elements are phosphatic tooth-like structures whose affinity and function is now believed to be part of the feeding apparatus of an extinct early vertebrate. Early ideas concluded that the conodontophorid was a soft bodied, bilaterally symmetrical nektonic organism, although there is still much debate concerning possible benthic, nektonic or combined mode of life. Conodont elements are composed of calcium carbonate fluorapatite with additional organic matter. They are found in marine deposits, commonly in black shales associated with graptolites, radiolarians, fish remains, brachiopods, cephalopods, trilobites and palaeocopid ostracods.

The name "conodont" was coined by C.H. Pander (a Russian) in 1856, who worked on Silurian fish fossils of Eastern Europe. Ulrich and Bassler (1926) described many new species from North America and were the first to recognise their biostratigraphic usefulness. In 1934 Schmidt and Scott discovered groups of individual elements preserved together on the same bedding plain. This importantly led to the theory that the individual elements were in life held in pairs (termed an apparatus) often likened to mouth parts. From the 1960's onwards conodonts have developed into one of the most important biostratigraphic tools available in Palaeozoic and Triassic rocks.
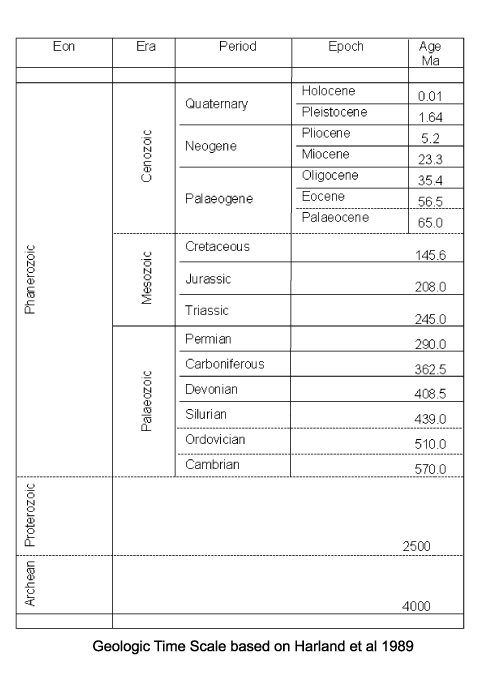
The very earliest conodonts are known from rocks of probable Precambrian age in Siberia, they are found more commonly in Cambrian deposits, diversity increased in the Ordovician and again during the Devonian. The conodont-bearing organism clearly survived the Permo-Triassic boundary extinctions but became extinct during the late Triassic. It has been noted that the extinction of the conodonts coincides with the diversification of dinoflagellates and first appearance of calcareous nannofosils. The most primitive conodonts are single cones, which dominate early Ordovician assemblages and reach a peak in the Arenigian (late Early Ordovician). The first platform type conodonts occur around this time as well. Conodont diversity and abundance declined in the Silurian. During the early and mid Devonian diversity gradually increased, reaching an acme in the late Devonian. In the early Carboniferous conodonts remained abundant and widespread but diversity decreased during the late Carboniferous. In the Permian the conodonts almost became extinct, however, they made a recovery in the early to middle Triassic only to disappear in the late Triassic.
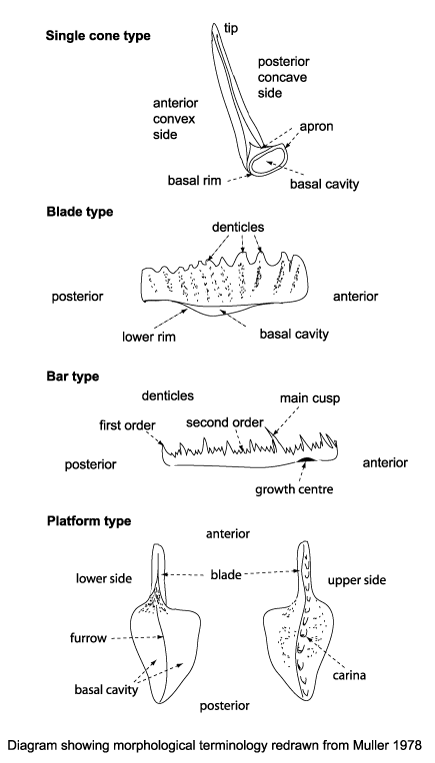
Conodonts have been assigned to their own Phylum, Conodonta, divided into two Orders based on chemical and ultrastructure differences. Eleven superfamilies have been recognised by reconstructing associations of individual elments into apparatuses; and morphological and element compositonal differences further divide these into forty seven families. One hundred and eighty genera have been recognised. It must be remembered that any classification of conodonts is an un-natural one, as it is based on morphology only. Morphologically, four main groups of conodonts can be distinguished.
- Simple cones: formed by a single tooth, or denticle.
- Blade-type: elongate, laterally compressed units formed by a row of denticles which are fused except at their tips.
- Bar-type: thin bars with or without a bent shaft which is commonly branched.
- Platform: it is thought these forms evolved from bar and blade-type conodonts by the developement of broad flanges into plates.
The fact that conodonts are relatively common in rocks of Palaeozoic age, a period when other microfossil groups are either not present or scarce, has made them extremely useful stratigraphic tools. Together with acritarchs, chitinozoa and spores, conodonts are the primary microfossils available to palaeontologists working on Ordovician to Permo-Triassic strata.
Isolated conodonts are widespread and abundant. Untill the nineteen eighties their biological affinities were still not known. Two enlightening fossil finds provided a few clues to the affinity of conodonts. The first, a chordate animal with conodonts scattered within what is interpreted as its gut from the fish bearing Namurian (Carboniferous) Bear Gulch limestone of Montana. The second, from the famous Cambrian
Burgess Shale of British Columbia, is a flattened worm-like animal 60mm long with a distinct head bearing a U-shaped structure interpreted as a lophophore (a circular or horseshoe shaped fleshy ridge surrounding the mouth, bearing tentacles found in Bryozoans and Brachiopods). At the base of each of the 20-25 tentacles is a compressed cone closely resembling some contemporaneous conodonts. However, the discovery of a Carboniferous fossil near Edinburgh (and subsequent finds in South Africa) has finaly solved the mystery of what the conodont elements are. It is now believed that they are the tooth-like feeding apparatus of a hagfish-like vertebrate. The co-occurrence of conodont elements in symmetrical pairs has allowed certain inferences to be made: The host animal probably exhibited bilateral symmetry. Several pairs of one sort can be associated with one or more pairs of another sort. The shape and arrangement of conodont elements in the apparatuses suggest that they were tooth-like feeding tools. The use of scanning electron microscopy has revealed signs of wear on conodont elements and it is thought that the host organism probably produced only one set in its life time.
Clearly very little can be stated about possible life cycles since the host organism of conodonts (conodontophorid) is extinct.
Since conodonts are resistant to mechanical and chemical attack preparation techniques can utilise acids such as acetic, formic, or monochloric to release the elements from their host rocks, which are commonly carbonates. Conodonts are commonly between 200 microns and 5 millimeters in size and can be sieved from finer materials and further concentrated by heavy liquid or ultrasonic techniques.
The cleaned specimens can then be viewed using a reflected light microscope and manipulated and mounted in slides in the same manner as foraminifera. Conodonts can also be observed in thin sections.
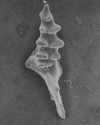
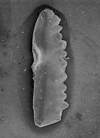
The following images are of a representative selection of conodonts aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and if possible a species name followed by the formation and the site location from which the sample was obtained. SEM images in all cases, courtesy of Leicester University
<
Diatoms
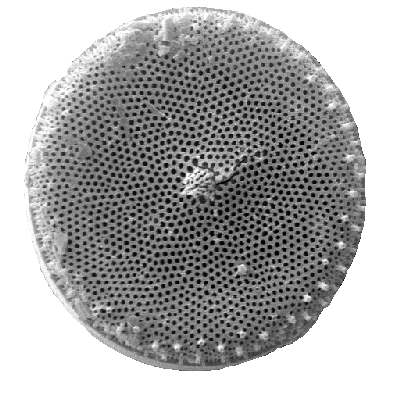
Diatoms are photosynthesising algae, they have a siliceous skeleton (frustule) and are found in almost every aquatic environment including fresh and marine waters, soils, in fact almost anywhere moist. They are non-motile, or capable of only limited movement along a substrate by secretion of mucilaginous material along a slit-like groove or channel called a raphe. Being autotrophic they are restricted to the photic zone (water depths down to about 200m depending on clarity). Both benthic and planktic forms exist. Diatoms are formally classified as belonging to the Division Chrysophyta, Class Bacillariophyceae. The Chrysophyta are algae which form endoplasmic cysts, store oils rather than starch, possess a bipartite cell wall and secrete silica at some stage of their life cycle. Diatoms are commonly between 20-200 microns in diameter or length, although sometimes they can be up to 2 millimeters long. The cell may be solitary or colonial (attached by mucous filaments or by bands into long chains). Diatoms may occur in such large numbers and be well preserved enough to form sediments composed almost entirely of diatom frustules (diatomites), these deposits are of economic benefit being used in filters, paints, toothpaste, and many other applications.
Diatoms have been studied since the late eighteenth century, however the first real advances in the field came in the early nineteenth century when diatoms were popular with microscopists utilising the emerging improvements in microscope resolution. Several European workers produced hand illustrated monographs on diatoms in the late nineteenth century. Notable amongst these are the works of Cleve, Ehrenberg, Grunow, Schmidt and Van Heurck. In the early twentieth century fossil diatoms were first studied and, most famously, Hustedt (1927-66) produced a taxonomic and ecological study of diatoms which remains a key reference today. Perhaps the most complete treatment of diatoms is that of Round et al. (1990).
First recorded occurrences of diatoms are from the Jurassic, however, these are uncertain and the earliest recorded well preserved diatoms are centric forms from the Aptian-Albian stages of the Cretaceous. Since these are quite diverse assemblages it is assumed diatoms have an earlier evolutionary history, perhaps lacking a relatively robust silica frustule. As with coccoliths, the earliest forms in the fossil record may reflect a biomineralisation event rather than an evolutionary appearance. The earliest araphid (lacking a raphe) pennate diatoms appear in the Late Cretaceous, and raphid pennates in the Middle Eocene. The earliest freshwater diatoms appear in the Palaeocene in Russia and the Late Eocene in North America. In a similar manner to Radiolaria, it has been noticed that there has been a gradual progression towards less heavily silicified frustules, probably as a result of increasing competition for a limited resource (silica).
Diatoms are divided into two Orders. The Centrales (now called the Biddulphiales) which have valve striae arranged basically in relation to a point, an annulus or a central areola and tend to appear radially symmetrical, and the Pennales (now called Bacillariales) which have valve striae arranged in relation to a line and tend to appear bilaterally symmetrical. The valve face of the diatom frustule is ornamented with pores (areolae), processes, spines, hyaline areas and other distinguishing features. It is these skeletal features which are used to classify and describe diatoms, which is an advantage in terms of palaeontology since the same features are used to define extant species as extinct ones. The classification system developed by Simonsen (1979) and further developed by Round et al. (1990) is currently the most commonly accepted. Diatoms commonly found in the marine plankton may be divided into the centric diatoms including three sub-orders based primarily on the shape of the cells, the polarity and the arrangement of the processes. These are the Coscinodiscineae, with a marginal ring of processes and no polarity to the symmetry, the Rhizosoleniineae with no marginal ring of processes and unipolar symmetry, and the Biddulphiineae with no marginal ring of processes and bipolar symmetry. The pennate diatoms are divided into two sub-orders, the Fragilariineae which do not posses a raphe (araphid) and the Bacillariineae which posses a raphe.
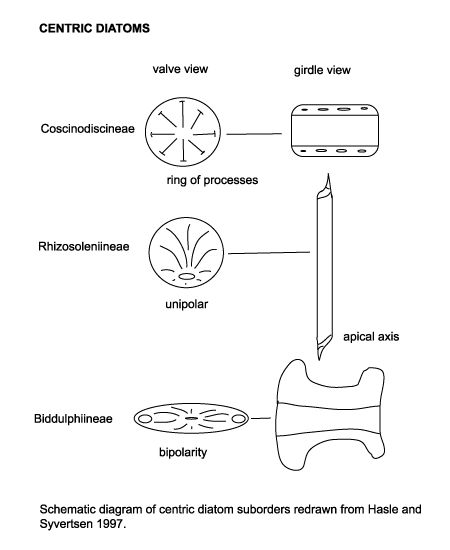
The evolutionary history of diatoms has been punctuated by several floristic turnovers, these have been utilised to allow basin wide biostratigraphic correlations. Diatoms are also used extensively in palaeoenvironmental studies particularly in palaeoceanography. Dissolution of diatom frustules during descent through the water column, on the sediment surface and during diagenesis may seriously alter the preserved assemblage by preferentialy dissolving more lightly silicified forms. High alkalinity of pore waters and burial temperatures in excess of 50 degrees centigrade are also known to increase dissolution of silica. Incorporation into faecal pellets or muciligenous aggregations, rapid burial and the formation of heavily silicified resting spores tend to counteract these problems, however, in marine samples it is thought that only 1% to 5% of the living assemblage in surface plankton is represented in the death assemblage found on the sediment surface. Despite these problems diatoms are still a useful and to a certain extent under-utilised group in terms of biostratigraphy.
Living diatoms often have specific salinity, temperature and other environmental tolerences, this together with the fact that a high proportion of fossil genera and species are still extant, makes it possible to use transfer functions to produce accurate palaeonvironmental reconstructions. This type of work has been used extensively and very successfuly, particularly in palaeolimnology,and the ">Environmental Change Research Centre at University College London is at the forefront of such research. The Deep Sea Drilling Project (now the "> Ocean Drilling Program ) has recovered many kilometers of cores and allowed the construction of a diatom biostratigraphy for most of the Cenozoic. Diatoms are particularly advantageous for biostratigraphic studies of high latitude sediments where calcareous microfossils are often poorly preserved, sparse, or of low diversity.
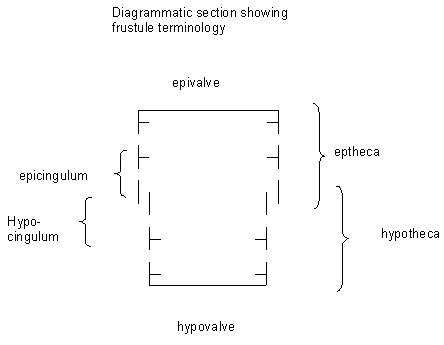
Diatoms have been well studied both in their natural habitat and in cultures by biologists and there is therefore a wealth of knowledge on their biology and ecology. The protoplast of diatoms consists of a cytoplasmic layer that lines the interior of the frustule and surrounds a large central vacuole, within the cytoplasmic layer there is a diploid nucleus and two to several pigment-bearing plastids (the site of photosyntheseis). The diatom frustule is often likened to a pill-box or agar dish with an epitheca (larger upper valve), and a hypotheca (smaller lower valve). The vertical lip or rim of the epitheca is called the epicingulum, and the epicingulum fits over (slightly overlaps) the hypocingulum of the hypotheca. The epicingulum and hypocingulum with one or several connective bands make up the girdle. Many diatoms are heterovalvate, i.e., the two valves of the frustule are dissimilar. This is most obvious within the family Achnanthaceae where one valve has a raphe and the other does not, and the Cymatosiraceae where one valve has a tubular process and the other does not. Chain-forming species with cells linked together by siliceous structures may, in addition, have separation valves. These valves are morphologically different from the valves within the chain. Therefore, one species may have four morphologically distinct types of valves.
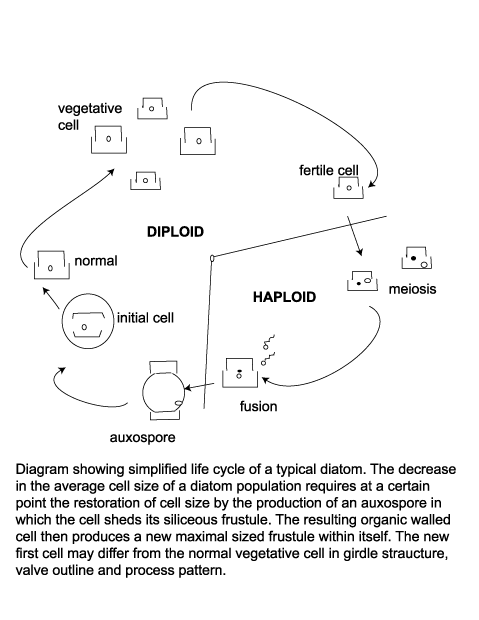
When a cell divides each new cell takes as its epitheca a valve of the parent frustule, and within ten to twenty minutes builds its own hypotheca; this process may occur between one and eight times per day. Availability of dissolved silica limits the rate of vegetative reproduction, but also because this method progressively reduces the average size of the diatom frustule in a given population there is a certain threshold at which restoration of frustule size is neccesary. Auxospores are then produced, which are cells that posses a different wall structure lacking the siliceous frustule and swell to the maximum frustule size. The auxospore then forms an initial cell which froms a new frustule of maximum size within itself. Many neritic planktonic diatoms alternate between a vegetative reproductive phase and a thicker walled resting cyst or statospore stage. The siliceous resting spore commonly forms after a period of active vegetative reproduction when nutrient levels have been depleted. Statospores may remain entirely within the the parent cell, partially within the parent cell or be isolated from it. An increase in nutreint levels and/or length of daylight cause the statospore to germinate and return to its normal vegatative state. Seasonal upwelling is therefore a vital part of many diatoms life cycle as a provider of nutrients and as a transport mechanism which brings statospores or their vegetative products up into the photic zone.
The resting spore morphology of some species is similar to that of the corresponding vegetative cell, whereas in other species the resting spores and the vegetative cells differ strongly. The two valves of a resting spore may be similar or distinctly different. Often the first valve formed is more similar to the valves of the vegetative cells than the second valve. This diversity of the valve types belonging to the same species calls for caution in identification work using cleaned diatom material.
Diatoms are easily prepared for veiwing using a light microscope. Wet samples can be smeared onto a slide for immediate examination and determination of possible further treatments. Organic matter may obscure the detail of the frustule so this is commonly removed using hydrogen peroxide or some other oxidising agent. A small amount of hydrochloric acid may be added to remove any calcium carbonate and the sample then rinsed in distilled water until free of acids. The sample can then be dilluted and strewn onto coverslips, dried and mounted on slides. Because the refractive indices of water and silica are very similar, a mounting medium with a higher refractive index is used in order to increase the contrast. An excellent guide to preparing diatom slides is provided by the Geography Department at UCL.
Diatom preparations may be observed using brightfield as well as phase contrast settings on a light microscope, the later is better for veiwing lightly silicified genera. Times forty dry or times one hundred oil-immersion objectives are most commonly used. The use of the scanning electron microscope allows the differentiation of processes if the inside of the valve can be veiwed. This is of particular use when attempting to speciate some of the centric diatoms. A guide to microscopy in relation to diatom preparations is provided by the Geography Department at UCL.
The following images are of a representative selection of diatoms aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and if possible a species name followed by its age range.
Foraminifera
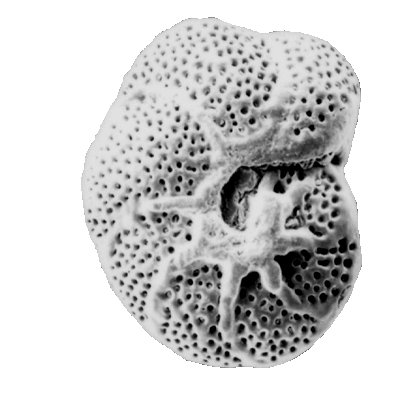
Foraminifera have a geological range from the earliest Cambrian to the present day. The earliest forms which appear in the fossil record (the allogromiine) have organic test walls or are simple agglutinated tubes. The term "agglutinated" refers to the tests formed from foreign particles "glued" together with a variety of cements. Foraminifera with hard tests are scarce until the Devonian, during which period the fusulinids began to flourish culminating in the complex fusulinid tests of the late Carboniferous and Permian times; the fusulinids died out at the end of the Palaeozoic. The miliolids first appeared in the early Carboniferous, followed in the Mesozoic by the appearance and radiation of the rotalinids and in the Jurassic the textularinids. The earliest forms are all benthic, planktic forms do not appear in the fossil record until the Mid Jurassic in the strata of the northern margin of Tethys and epicontinental basins of Europe. They were probably meroplanktic (planktic only during late stages of their life cycle). The high sea levels and "greenhouse" conditions of the Cretaceous saw a diversification of the planktic foraminifera, and the major extinctions at the end of the Cretaceous included many planktic foraminifera forms. A rapid evolutionary burst occurred during the Palaeocene with the appearance of the planktic globigerinids and globorotalids and also in the Eocene with the large benthic foraminifera of the nummulites, soritids and orbitoids. The orbitoids died out in the Miocene, since which time the large foraminifera have dwindled. Diversity of planktic forms has also generally declined since the end of the Cretaceous with brief increases during the warm climatic periods of the Eocene and Miocene.
History of Study
The study of foraminifera has a long history, their first recorded "mention" is in Herodotus (fifth century BC) who noted that the limestone of the Egyptian pyramids contained the large benthic foraminifer Nummulites. In 1835 Dujardin recognised foraminifera as protozoa and shortly afterwards d'Orbigny produced the first classification. The famous 1872 HMS Challenger cruise , the first scientific oceanographic research expedition to sample the ocean floor collected so many samples that several scientists, including foraminiferologists such as H.B. Brady were still working on the material well in to the 1880's. Work on foraminifera continued throughout the 20th century, workers such as Cushman in the U.S.A and Subbotina in the Soviet Union developed the use of foraminifera as biostratigraphic tools. Later in the 20th century Loeblich and Tappan and Bolli carried out much pioneering work.
Range
Foraminifera have a geological range from the earliest Cambrian to the present day. The earliest forms which appear in the fossil record (the allogromiine) have organic test walls or are simple agglutinated tubes. The term "agglutinated" refers to the tests formed from foreign particles "glued" together with a variety of cements. Foraminifera with hard tests are scarce until the Devonian, during which period the fusulinids began to flourish culminating in the complex fusulinid tests of the late Carboniferous and Permian times; the fusulinids died out at the end of the Palaeozoic. The miliolids first appeared in the early Carboniferous, followed in the Mesozoic by the appearance and radiation of the rotalinids and in the Jurassic the textularinids. The earliest forms are all benthic, planktic forms do not appear in the fossil record until the Mid Jurassic in the strata of the northern margin of Tethys and epicontinental basins of Europe. They were probably meroplanktic (planktic only during late stages of their life cycle). The high sea levels and "greenhouse" conditions of the Cretaceous saw a diversification of the planktic foraminifera, and the major extinctions at the end of the Cretaceous included many planktic foraminifera forms. A rapid evolutionary burst occurred during the Palaeocene with the appearance of the planktic globigerinids and globorotalids and also in the Eocene with the large benthic foraminifera of the nummulites, soritids and orbitoids. The orbitoids died out in the Miocene, since which time the large foraminifera have dwindled. Diversity of planktic forms has also generally declined since the end of the Cretaceous with brief increases during the warm climatic periods of the Eocene and Miocene.

Classification
Foraminifera are classified primarily on the composition and morphology of the test. Three basic wall compositions are recognised, organic (protinaceous mucopolysaccharide i.e. the allogromina), agglutinated and secreted calcium carbonate (or more rarely silica). Agglutinated forms, i.e the Textulariina, may be composed of randomly accumulated grains or grains selected on the basis of specific gravity, shape or size; some forms arrange particular grains in specific parts of the test. Secreted test foraminifera are again subdivided into three major groups, microgranular (i.e. Fusulinina), porcelaneous (i.e. Miliolina) and hyaline (i.e. Globigerinina). Microgranular walled forms (commonly found in the late Palaeozoic) are composed of equidimensional subspherical grains of crystalline calcite. Porcelaneous forms have a wall composed of thin inner and outer veneers enclosing a thick middle layer of crystal laths, they are imperforate and made from high magnesium calcite. The hyaline foraminifera add a new lamella to the entire test each time a new chamber is formed; various types of lamellar wall structure have been recognised, the wall is penetrated by fine pores and hence termed perforate. A few "oddities" are also worth mentioning, the Suborder Spirillinina has a test constructed of an optically single crystal of calcite, the Suborder Silicoloculinina as the name suggests has a test composed of silica. Another group (the Suborder Involutina) have a two chambered test composed of aragonite. The Robertinina also have a test composed of aragonite and the Suborder Carterina is believed to secrete spicules of calcite which are then weakly cemented together to form the test.
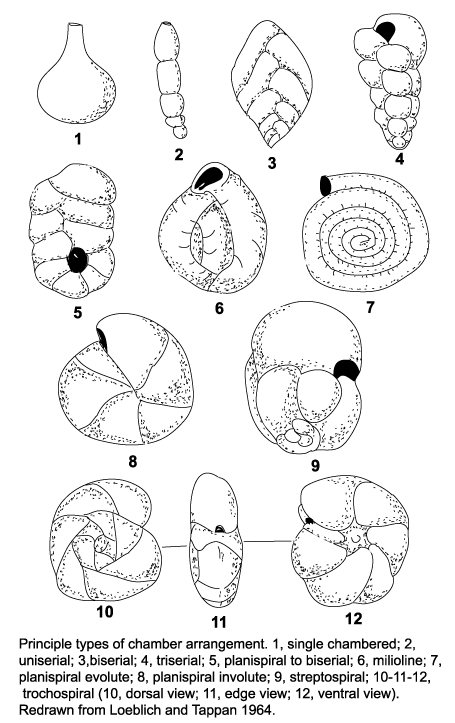
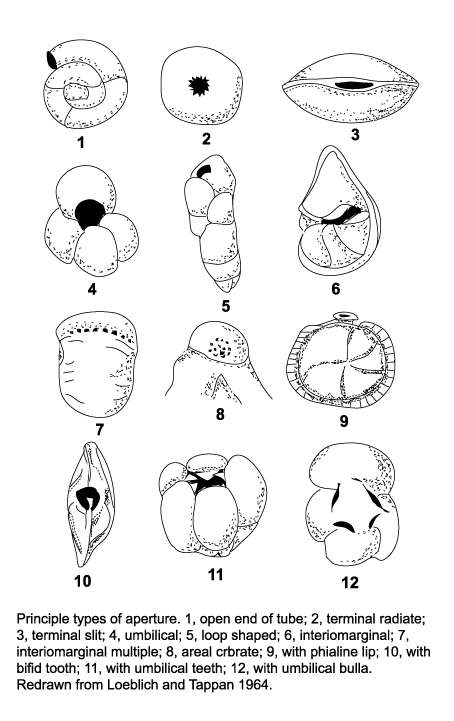
The morphology of foraminifera tests varies enormously, but in terms of classification two features are important. Chamber arrangement and aperture style, with many subtle variations around a few basic themes. These basic themes are illustrated in the following two diagrams but it should be remembered that these are only the more common forms and many variations are recognised.
Applications
As previously mentioned, foraminifera have been utilised for biostratigraphy for many years, and they have also proven invaluable in palaeoenvironmental reconstructions most recently for palaeoceanographical and palaeoclimatological purposes. For example palaeobathymetry, where assemblage composition is used and palaeotemperature where isotope analysis of foraminifera tests is a standard procedure. In terms of biostratigraphy, foraminifera have become extremely useful, different forms have shown evolutionary bursts at different periods and generally if one form is not available to be utilised for biostratigraphy another is. For example preservation of calcareous walled foraminifera is dependent on the depth of the water column and Carbonate Compensation Depth (the depth below which dissolution of calcium carbonate exceeds the rate of its deposition), if calcareous walled foraminifera are therefore not preserved agglutinated forms may be. The oldest rocks for which foraminifera have been biostratigraphically useful are Upper Carboniferous to Permian strata, which have been zoned using the larger benthic fusulinids. Planktic foraminifera have become increasingly important biostratigraphic tools, especially as petroleum exploration has extended to offshore environments of increasing depths. The first and last occurrence of distinctive "marker species" from the Cretaceous to Recent (particularly during the Upper Cretaceous) has allowed the development of a well established fine scale biozonation. Benthic foraminifera have been used for palaeobathymetry since the 1930's and modern studies utilise a variety of techniques to reconstruct palaeodepths. For studies of relatively recent deposits simple comparison to the known depth distribution of modern extant species is used. For older material changes in species diversity, planktic to benthic ratios, shell-type ratios and test morpholgy have all been utilised. Variations in the water temperature inferred from oxygen isotopes from the test calcite can be used to reconstruct palaeoceanographic conditions by careful comparison of changes in oxygen isotope levels as seen in benthic forms (for bottom waters) and planktic forms(for mid to upper waters). This type of study has allowed the reconstruction of oceanic conditions during the Eocene-Oligocene, the Miocene and the Quaternary. Benthic foraminifera have been divided into morphogroups based on the test shape and these groups used to infer palaeo-habitats and substrates; infaunal species tending to be elongate and streamlined in order to burrow into the substrate and epifaunal species tending to be more globular with one relatively flatter side in order to facilitate movement on top of the substrate. It should be remembered, however, that a large variety of morphologies and possible habitats have been recognised making such generalisations of only limited use. Studies of modern foraminifera have recognised correlations between test wall type (for instance porcelaneous, hyaline, agglutinated), palaeodepths and salinity by plotting them onto triangular diagrams.
Biology
Studies of living foraminifera, in controlled laboratory environments, have provided limited information regarding trophic strategies but much has been inferred by relating test morphology to habitat. Foraminifera utilise a huge variety of feeding mechanisms, as evidenced by the great variety of test morphologies that they exhibit. From the variety of trophic habits and test morphologies a few generalisations may be made. Branching benthic foraminifera such as Notodendrodes antarctikos ,which resembles a microscopic tree, absorbs dissolved organic matter via a "root" system. Other sessile benthic foraminifera exhibit test morphologies dependent on the substrate on or in which they live, many are omnivorous opportunistic feeders and have been observed to consume autotrophic and heterotrophic protists (including other foraminifera), metazoans and detritus. Some suspension feeding foraminifera utilise their pseudopodia to capture food from the water column, or interstitial pore waters, Elphidium crispum forms a "spiders web" between the stipes of coralline algae. Infaunal forms are probably detritivores and commonly have elongate tests to facilitate movement through the substrate. Benthic and planktonic foraminifera which inhabit the photic zone often live symbiotically with photosynthesising algae such as dinoflagellates, diiatoms and chlorophytes. It is thought the large benthic, discoidal and fusiform foraminifera attain their large size in part because of such associations. Foraminifera are preyed upon by many different organisms including worms, crustacea, gastropods, echinoderms, and fish. It should be remembered that the biocoenosis (life assemblage) will be distorted by selectivedestruction by predators.
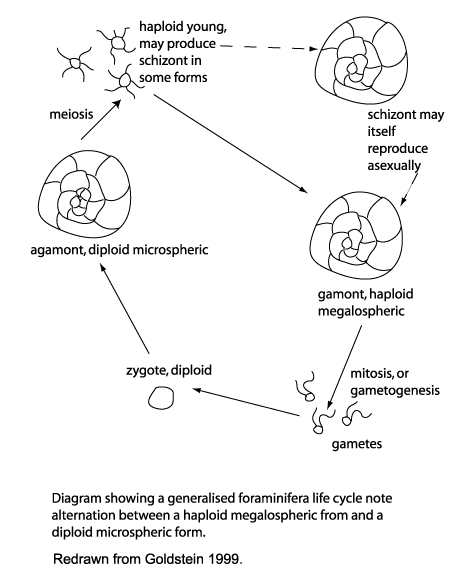
Life Cycle
Of the approximately 4000 living species of foraminifera the life cycles of only 20 or so are known. There are a great variety of reproductive, growth and feeding strategies, however the alternation of sexual and asexual generations is common throughout the group and this feature differentiates the foraminifera from other members of the Granuloreticulosea. An asexually produced haploid generation commonly form a large proloculus (initial chamber) and are therefore termed megalospheric. Sexually produced diploid generations tend to produce a smaller proloculus and are therefore termed microspheric. Importantly in terms of the fossil record, many foraminiferal tests are either partially dissolved or partially disintegrate during the reproductive process.The planktonic foraminifera Hastigerina pelagica reproduces by gametogenesis at depth, the spines, septa and apertural region are resorbed leaving a tell-tale test. Globigerinoides sacculifer produces a sac-like final chamber and additional calcification of later chambers before dissolution of spines occurs, this again produces a distinctive test, which once gametogenesis is complete sinks to the sea bed.
Preparation Techniques
WARNING: Please remember all preparation techniques require the use of hazardous materials and equipment and should only be carried out in properly equiped laboratories, wearing the correct safety clothing and under the supervision of qualified staff. Foraminifera range in size from several millimeters to a few tens of microns and are preserved in a variety of rock types. The preparation techniques used depend on the rock type and the "predicted" type of foraminifera one expects to find. Very hard rocks such as many limestones are best thin sectioned as in normal petrological studies, except instead of grinding to a set thickness (commonly 30 microns) the sample is ground very carefully by hand until the optimum thickness is obtained for each individual sample. This is a skilled job and requires expensive equipment but provides excellent results and is particularly used in the study of larger benthic foraminifera from reef type settings. Planktic and smaller benthic foraminifera are prepared by crushing the sample into roughly five millimeter fragments. The crushed sample is then placed in a strong glass beaker or similar vessel and water and washing soda or 6% hydrogen peroxide added, left to stand and then heated and allowed to simmer. The length of time the sample is left to simmer depends on the rock type involved and if peroxide is used the sample should not be left immersed in the solution for more than about half an hour. Next, the material is washed through a 63 micron sieve untill the liquid coming through the sieve is clean (i.e. the clay fraction has been removed). The sample can then be dried and sieved into fractions (generally 63-125 microns, 125-250 microns, 250-500 microns and greater than 500 microns) using a "nest" of dry sieves. Care must be taken to clean all sieves and materials used between the preparation of each sample to prevent contamination.
Observation Techniques
Thin sections are veiwed using transmitted-light petrological type microscopes. Washed, dried fossil samples can be picked from any remaining sediment using a fine brush and a reflected light, binocular microscope. The best method is to scatter a fine dusting of sieved sediment on to a black tray divided into squares, this can then be scanned under the microscope and any foraminifera preserved in the sediment can be picked out with a fine brush (preferably a 000 sable-haired brush). The picked specimens can then be mounted in card slides divided into numbered squares with sliding glass covers. Gum tragocanth was traditionally used to attach the specimens to the slides but modern office-type paper adhesives are now used.
Images
The following images are of a representative selection of foraminifera aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and, if possible, a species name followed by its age range, the site location from which the sample was obtained and its size in microns. LM (Light Microscope) SEM (Scanning Electron Microscope) TS (Thin Section). Typical and selected marker species are illustrated from each main period of the geological column in which foraminifera occur. Because Foraminifera formsuch a diverse taxon they have been split into three groups: planktics, benthics and larger benthics. Planktic Foraminifera
Benthic Foraminifera
Larger Benthic Foraminifera
Planktic |
|
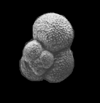
| Globigerina bulloides d'Orbigny |
| Pliocene-Recent |
| South Africa |
| 380 microns spiral view SEM |
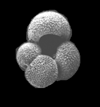
|
| Globigerina bulloides d'Orbigny |
| Pliocene-Recent |
| South Africa |
| 416 microns umbilical view SEM |
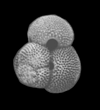
|
| Globigerinoides ruber d'Orbigny |
| Miocene-Recent |
| South Africa |
| spiral view SEM |
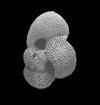
|
| Globigerinoides sacculifer (Brady) |
| Miocene-Recent |
| South Africa |
| spiral view SEM |
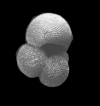
|
| Globigerinoides sacculifer (Brady) |
| Miocene-Recent |
| South Africa |
| umbilical view SEM |
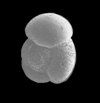
|
| Globorotalia inflata d'Orbigny |
| Pliocene-Recent |
| South Africa |
| spiral view SEM |
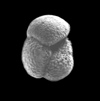
|
| Globorotalia inflata d'Orbigny |
| Pliocene-Recent |
| South Africa |
| umbilical view SEM |
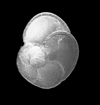
|
| Globorotalia menardii (Parker, Jones and Brady) |
| Pliocene-Recent |
| South Africa |
| spiral view SEM |
|
| Globorotalia menardii (Parker, Jones and Brady) |
| Pliocene-Recent |
| South Africa |
| umbilical view SEM |

|
| Neogloboquadrina pachyderma (Ehrenberg) |
| Pliocene-Recent |
| South Africa |
| spiral view SEM |
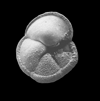
|
| Neogloboquadrina pachyderma (Ehrenberg) |
| Pliocene-Recent |
| South Africa |
| umbilical view SEM |

|
| Orbulina universa d'Orbigny |
| Middle Miocene-Recent |
| South Africa |
| SEM |

|
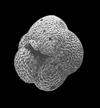
| Hantkenina alabamensis Cushman, 1927 |
| Eocene |
| Montgomery Landing, Red River, Louisiana, USA |
| side view (slightly broken specimen) SEM |
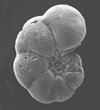
|
| Pseudohastigerina micra (Cole, 1927) |
| Eocene-Oligocene |
| Montgomery Landing, Red River, Louisiana, USA |
| side view SEM |
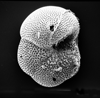
|
| Globorotalia centralis Cushman and Bermudez, 1937 |
| Eocene |
| Montgomery Landing, Red River, Louisiana, USA |
| umbilical view SEM |
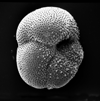
|
| Globorotalia cerro-azulensis Cole, 1928 |
| Eocene |
| Montgomery Landing, Red River, Louisiana, USA |
| umbilical view SEM |
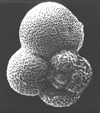
|
| Parasubbotina varianta (Subbotina, 1953) |
| Lower-Middle Palaeocene |
| Zin Valley, Israel |
| spiral view SEM |
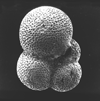
|
| Parasubbotina varianta (Subbotina, 1953) |
| Lower-Middle Palaeocene |
| Zin Valley, Israel |
| umbilical view SEM |
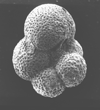
|
| Parasubbotina pseudobulloides (Plummer, 1926) |
| Lower-Middle Palaeocene |
| Zin Valley, Israel |
| umbilical view SEM |
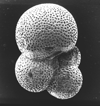
|
| Subbotina triloculinoides (Plummer, 1926) |
| lower-upper Palaeocene |
| Zin Valley, Israel |
| umbilical view SEM |
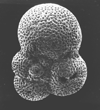
|
| Subbotina triloculinoides (Plummer, 1926) |
| Palaeocene |
| Zin Valley, Israel |
| spiral view SEM |

|
| Abathomphalus mayaroensis (Bolli) |
| Upper Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| umbilical view SEM |

|
| Abathomphalus mayaroensis (Bolli) |
| Upper Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| lateral view SEM |

|
| Contusotruncana contusa (Cushman) |
| Upper Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| dorsal view SEM |

|
| Contusotruncana contusa (Cushman) |
| Upper Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| ventral view SEM |

|
| Globotruncana linneiana (d'Orbigny) |
| Santonian-Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| dorsal view SEM |

|
| Globotruncana linneiana (d'Orbigny) |
| Santonian-Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| ventral view SEM |
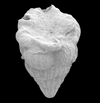
|
| Racemiguembelina fructicosa (Egger) |
| Middle-Uppper Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| SEM |

|
| Racemiguembelina fructicosa (Egger) |
| Middle-Uppper Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| SEM |
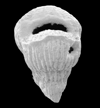
|
| Pseudotextularia elegans (Rzehak) |
| Campanian-Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| SEM |

|
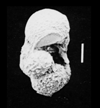
| Pseudoguembelina excolata (Cushman) |
| Campanian-Maastrichtian (Upper Cretaceous) |
| Kassbah, N.W. Syria |
| SEM |
|
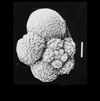
| Archaeoglobigerina cretacea (d'Orbigny) |
| Coniacian-Maastrichtian (Upper Cretaceous) |
| Sens, N. France |
| scale bar 100 microns edge view SEM |
|
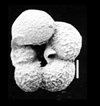
| Archaeoglobigerina cretacea (d'Orbigny) |
| Coniacian-Maastrichtian (Upper Cretaceous) |
| Sens, N. France |
| scale bar 100 microns dorsal view SEM |
|
| Archaeoglobigerina cretacea (d'Orbigny) |
| Coniacian-Maastrichtian (Upper Cretaceous) |
| Sens, N. France |
| scale bar 100 microns ventral view SEM |
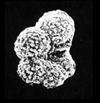
|
| Hedbergella delrioensis (Carsey) |
| Coniacian-Santonian (Upper Cretaceous) |
| Faircross, UK |
| scale bar 100 microns ventral view SEM |
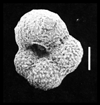
|
| Whiteinella baltica Douglas and Rankin |
| Coniacian-Santonian (Upper Cretaceous) |
| Winterbourne, UK |
| scale bar 100 microns ventral view SEM |
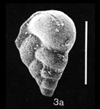
|
| Heterohelix pulchra (Brotzen) |
| Coniacian-Maastrichtian (Upper Cretaceous) |
| N. Norfolk, UK |
| scale bar 100 microns side view SEM |

|
| Heterohelix globulosa (Ehrenberg) |
| Coniacian-Maastrichtian (Upper Cretaceous) |
| Sens, N. France |
| scale bar 100 microns side view SEM |
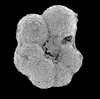
|
| Hedbergella planispira (Tappan) |
| Aptian-Coniacian (Upper Cretaceous) |
| Karai, S.E. India |
| ventral view SEM |

|
| Hedbergella sigali Moullade |
| Barremian-Aptian (Lower Cretaceous) |
| Karai, S.E. India |
| ventral view SEM |
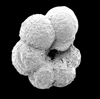
|
| Ticinella primula Luterbacher |
| Albian (Lower Cretaceous) |
| Karai, S.E. India |
| ventral view SEM |
| TOP |
Benthic |

|
| Spiroloculina ornata (d'Orbigny) |
| -Recent |
| Sea of Marmara |
| side view SEM |

|
| Elphidium macellum (Fichtel and Moll) |
| -Recent |
| Sea of Marmara |
| side view SEM |

|
| Brizalina alata (Seguenza) |
| -Recent |
| Sea of Marmara |
| side view SEM |
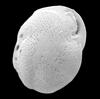
|
| Cassidulina neocarinata (Thalmann) |
| -Recent |
| Sea of Marmara |
| ventral view SEM |

|
| Siphotextularia concava (Karrer) |
| -Recent |
| Sea of Marmara |
| side view SEM |

|
| Bigenerina nodosaria (d'Orbigny) |
| ??-Recent |
| Sea of Marmara |
| side view SEM |

|
| Planorbulina mediterranensis (d'Orbigny) |
| ??-Recent |
| Sea of Marmara |
| unattached side SEM |

|
| Nonionella opima (Cushman) |
| ??-Recent |
| Sea of Marmara |
| side view SEM |
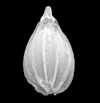
|
| Lagena striata (d'Orbigny) |
| ??-Recent |
| Sea of Marmara |
| side view SEM |
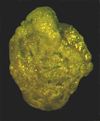
|
| Alveovalvulina suteri Bronnimann |
| Gulf of Mexico |
| LM |
|
| Cyclamina cancellata Brady |
| Gulf of Mexico |
| 1000 microns LM |
|
| Cyclamina acutidorsata (von Hantken) |
| Oligocene-Miocene |
| Gulf of Mexico |
| 1000 microns LM |

|
| Cyclamina acutidorsata (von Hantken) |
| Gulf of Mexico |
| 1000 microns LM |

|
| Reticulophragmium rotundidorsata (von Hantken) |
| Eocene-Miocene |
| Gulf of Mexico |
| 600 microns LM |
|
| Cribrostomoides sp. |
| Gulf of Mexico |
| 600 microns side view LM |

|
| Cribrostomoides sp. |
| Gulf of Mexico |
| 600 microns apertural view LM |

|
| Haplophragmoides bradyi (Robertson) |
| Gulf of Mexico |
| 600 microns side view LM |
|
| Haplophragmoides bradyi (Robertson) |
| Gulf of Mexico |
| 600 microns apertural view LM |

|
| Jarvisella karamatensis Bronnimann |
| Gulf of Mexico |
| 600 microns apertural view LM |
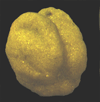
|
| Recurvoides azuamensis Bermudez |
| Oligocene? |
| Gulf of Mexico |
| 1500 microns apertural view LM |
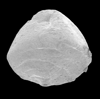
|
| Textularia tatumi Cushman and Ellisor |
| Miocene? |
| Gulf of Mexico |
| SEM |

|
| Valvulina flexilis Cushman and Renz |
| Oligocene-Miocene |
| Gulf of Mexico |
| SEM |

|
| Nodosaria sp. |
| Jurassic |
| Villers sur Mer, Normandy, France |
| SEM |

|
| Nodosaria sp. |
| Jurassic |
| Villers sur Mer, Normandy, France |
| close up of aperture SEM |

|
| Vaginulina bernardi Paalzow |
| Oxfordian (Jurassic) |
| Villers sur Mer, Normandy, France |
| SEM |
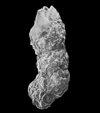
|
| Ammobaculites coprolithiformis Schwager |
| Bathonian-Kimmeridgian (Jurassic) |
| Villers sur Mer, Normandy, France |
| SEM |

|
| Psammosphaera sp. Schulze |
| middle Ordovician-Recent |
| Voring Basin, offshore Norway |
| SEM |
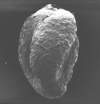
|
| Miliamina fusca (Bradey) |
| ?-Recent |
| Voring Basin, offshore Norway |
| SEM |
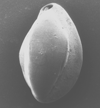
|
| Quinqueloculina impressa Reuss |
| ?-Eocene |
| Voring Basin, offshore Norway |
| SEM |
Larger Benthic |

|
| Helicolepidina cf nortoni Vaughan |
| ?Eocene |
| Barinas, S.W Venezuela |
| Thin Section |

|
| Heterostegina sp d'Orbigny |
| ?Eocene |
| Barinas, S.W Venezuela |
| TS |
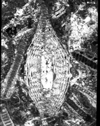
|
| Lepidocyclina tobleri panamensis (Cushman) |
| ?Eocene |
| Barinas, S.W Venezuela |
| TS |
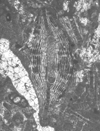
|
| Lepidocyclina pustulosa tobleri (Douville) |
| ?Eocene |
| Barinas, S.W Venezuela |
| TS |
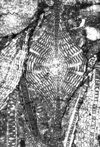
|
| Lepidocyclina pustulosa pustulosa (Douville) |
| ?Eocene |
| Barinas, S.W Venezuela |
| TS |
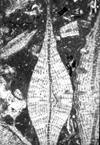
|
| Discocyclina (Discocyclina) marginata (Cushman) |
| ?Eocene |
| Barinas, S.W Venezuela |
| TS |
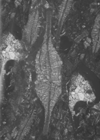
|
| Lepidocyclina cf tobleri (Cushman) |
| ?Eocene |
| Barinas, S.W Venezuela |
| TS |

|
| Nummulites sp. Lamarck |
| Paleocene-Holocene |
| Ainsa, Southern Pyrennees, Spain |
| Thin section |

|
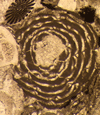
| Discocyclina sp. Gumbel |
| Mid Paleocene-Upper Eocene |
| Ainsa, Southern Pyrennees, Spain |
| Thin section |
|
| Alveolina sp. d'Orbigny |
| Upper Paleocene-Upper Eocene |
| Ainsa, Southern Pyrennees, Spain |
| Thin section |

|
| Alveolina sp. d'Orbigny |
| Upper Paleocene-Upper Eocene |
| Ainsa, Southern Pyrennees, Spain |
| Thin section |
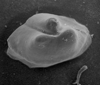
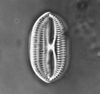
Ostracods

The Class Ostracoda is separated from other Crustacea by their laterally compressed body, undifferentiated head, seven or less thoracic limbs and the bivalved, perforate carapace lacking growth lines. The living ostracods are further classified in many cases by variations in their appendages and other soft parts. Although exceptionally well preserved fossil ostracods with the soft parts intact have been found these are very rare and therefore the morphological features (see below) of the carapace have become vital in fossil ostracod classification. The Ostracoda have been divided into five Orders, the extant Podocopida and Myodocopida and the extinct Phosphatocopida, Leperditicopida and Palaeocopida (however, the latter groups may well not be ostracods in the strict biological sense).
Since the fossil record of planktonic marine ostracods is so patchy, biostratigraphic uses of ostracods based on benthic forms are limited to specialised marine environments for example in the Jurassic of the North Sea. In the marine environment benthic ostracods are utilised for palaeoenvironmental reconstructions. Freshwater and brackish facies commonly contain abundant ostracods which are used for environmental studies and for biostratigraphic zonations, for instance in non-marine sediments from Mongolia and China.
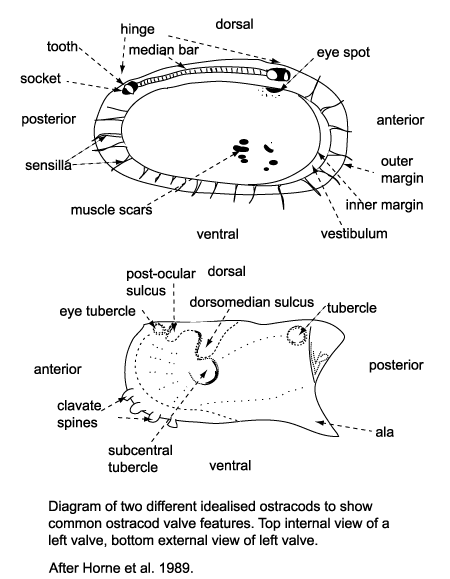
Several morphological features of ostracods are at times preserved in the fossil forms and have been utilised in their classification. The ostracod carapace is usually ovate, kidney-shaped or bean-shaped, it is divided into a right and left valve, one being, commonly slightly larger than the other partially overlapping it, and hinged at the dorsal margin. The hinge is an important feature in terms of taxonomy and classification. Four basic types of hinge are recognised:
- The adont hinge is the simplest, without teeth or sockets, often forms part of a contact groove on the larger valve and a corresponding ridge on the smaller valve.
- The merodont hinge is composed of a tooth and socket at each end of a groove or ridge structure (complementary negative and positive structures in left and right valves).
- The entomodont hinge differs from the merodont hinge style by having a coarsely crenulated anterior portion of the median groove/ ridge element.
- The amphidont hinge has a more complex median structure with an anterior tooth and socket.
Ostracods sense their surroundings using sensilla (hairs or bristles) which project through the carapace via pore canals, at the margins these are called marginal pore canals. Some shallow water ostracods have eyes and their carapaces have clear eyespots or raised eye tubercles.
Ostracods can reproduce sexually and asexually (parthenogenesis). Ostracods show sexual dimorphism, that is males and females of the same species have carapaces of differing size and shape.They grow by moulting up to nine growth stages (instars). Because of this sexual dimorphism and the ontogenetic variations of ostracods great care must be taken with taxonomy, as a single species may have a series of juvenile stages as well as two adult morphotypes.
The ecology of ostracods is often reflected in the shape and structure of their carapaces hence making them useful palaeoenvironmental indicators. Freshwater ostracods in general tend to have smooth, thin, weakly calcified simple bean-shaped carapaces. They feed on a wide range of food stuffs including diatoms, bacteria and detritus. Pelagic ostracods also tend to have thin, smooth shells and may have long powerful swimming appendages or antennules which have led to the formation of rostral incisures at the anterior of the carapace to allow freer movement of these appendages. Benthic ostracods are commonly detritivores or filter feeders, they either burrow into the substrate, in which case their carapaces tend to be smooth, small, robust and sometimes elongated. Epifaunal types may have flattened ventral surfaces sometimes with projecting alar wings, frills, keels or lateral spines. Those found on coarser substrates in higher energy environments tend to have more robust heavily ribbed or reticulated carapaces.
Ostracods like other Crustacea moult between growth stages (called an instar), this process is known as ecdysis. There are usually nine instars between egg and adult. This fact has extremely important implications for palaeontological studies. For example, if an assemblage contains a mix of instars it is relatively safe to assume the material is in situ (a biocoenosis, a true reflection of the living assemblage). Ostracods also have a variety of complex reproductive strategies, including brood care of eggs within the carapace (e.g. Darwinula), desiccation-resistant eggs (known to survive in a dry state only to hatch on immersion in water years later), sexual and asexual strategies including parthenogenesis which in Darwinula is thought to be the only method of reproduction utilised. Parthenogenesis is asexual reproduction via the female only, there appears to have been no male Darwinula's for many thousands if not millions of years!
Ostracod carapaces range in size from approximately 100 microns up to several millimetres, and they are commonly prepared in the same way as foraminifera with careful washing with hydrogen peroxide and/or washing soda and sieving using a standard 63 micron sieve. Several washes may be required to break down well indurated material and care should be taken when washing through the sieve to prevent breakage of the specimens. The cleaned residue can then be dried, sieved into fractions (generally 63-125 microns, 125-250 microns, 250-500 microns and greater than 500 microns) and "picked". Care must be taken to clean all sieves and materials used between the preparation of each sample to prevent contamination.
Once fossil samples have been prepared ostracod carapaces can be picked from any remaining sediment using a fine brush and a reflected light, binocular microscope. The best method is to scatter a fine dusting of sieved sediment on to a black tray divided into squares, this can then be scanned under the microscope and any ostracods preserved in the sediment can be picked out with a fine brush (preferably a 000 sable paint brush). The picked specimens can then be mounted in card slides divided into numbered squares with sliding glass covers. Gum tragocanth was traditionally used to attach the specimens to the slides but modern office type water soluble paper adhesives are now used. Ostracods are large enough to be observed live in wet preps under microscopes and sometimes with the naked eye. Almost any relatively still water will contain ostracods and samples can be collected especially by scraping them from the surface of water plants or sediment.
The following images are of a representative selection of ostracods aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and, if possible, a species name followed by its age range, the site location from which the sample was obtained and its size in microns. LM (Light Microscope) SEM (Scanning Electron Microscope). Typical and selected marker species are illustrated from each main period of the geological column in which ostracods occur. Click on an image to view a larger version. Because Ostracods are such a diverse taxa they have been split into two groups: freshwater and marine forms.
Palynology

The term Palynology, like "calcareous nannofossils", is a cover-all informal classification. It includes a large range of both extinct and extant organisms, the unifying character which allows us to "group" them together as palynomorphs is the composition of the preserved remains. This is usually an extremely tough, inert organic material called sporopollenin, although sometimes it may be chitin or pseudochitin. It must be remembered, however, that the preservation of all palynomorphs depends on the diagenetic processes they are subjected to.
Palynomorphs are particularly susceptible to degradation by oxidising agents. The term palynology is derived from the Greek for sprinkle, and was suggested by Hyde and Williams in 1944. Palynomorphs generaly fall into one of nine groups:
- Cryptarchs, a rather outdated term for a group of Pre-Cambrian spherical forms of uncertain affinity or origin.
- Acritarchs, an artificial group, coined by Evitt in 1963, (the name means "of uncertain origin") includes any small, organic walled microfossil which cannot be assigned to a particular group. They are characterised by varied sculpture, some being spiny and others smooth. They are believed to have algal affinities, probably being the cysts of planktonic eukaryotic algae. They are valuable Palaeozoic biostratigraphic and palaeoenvironmental tools.
- Chitinozoa, are large (50-2000 microns in length) flask shaped palynomorphs which appear dark and almost opaque when viewed using a light microscope. They are important Palaeozoic microfossils.
- Scolecodonts are the mouth parts of annelid worms they are composed of chitin and silica, the chitin being carbonised to jet black during fossilisation; they are of limited stratigraphic use.
- Green and blue-green algae, is another catch-all grouping which includes two important freshwater forms: Botryococcus and Pediastrum. It also encompasses the prasinophycean algae which include the widespread and seemingly environmentaly tolerant genera Tasmanites, Pterospermella and Cymatiosphaera. The Zygnemataceae are another long ranging (Carboniferous to Recent) freshwater algal group which usually occur in shallow oxygen-rich water.
- Spores and pollen, derived from terrestrial plants, are probably the most widespread and important palynomorphs. Because of the ease with which they are transported by wind and water they can be found in almost all environments and sedimentary deposits. Spores first appear towards the end of the Silurian (coincident with the first land ferns) and continue to the present, they are stratigraphicly important. Pollen first appears in the Carboniferous (coincident with the first gymnosperms, including conifers), they typically have a bisaccate form. The evolution of the flowering plants (angiosperms) in the Cretaceous brought further developments in pollen form.
Dinoflagellates are unicellular microplankton, they are commonly autotrophs but heterotrophic forms also occur, today they are second only to diatoms as marine primary producers. Dinoflagellates have a complex life cycle which includes a resting cyst stage, it is this cyst which is preserved (correctly termed a dinocyst). They are extremely important biostratigraphic and palaeoenvironmental tools especially in the Mesozoic and Cenozoic. They are composed of dinosporin, similar to sporopollenin but peculiar to dinoflagellates. The name derives from the Greek "dinos" meaning whirling and refers to the characteristic flagella, which propels them in a spiral motion.
Fungal bodies are present in many palynological preparations, and appear as smooth, medium brown, rather simple bodies. They range from the Pre-Cambrian to the present so are of limited use stratigraphically.
Foraminiferal test linings are common in palynology preparations from marine samples, but because they cannot be assigned to particular genera they are of little use although they have been used for palaeoenvironmental interpretations.
A whole branch of palynology called palynofacies has developed based on the study of the total kerogen content of a sample. The thermal state of the organic matter, as well as composition of both amorphous matter and palynomorphs is taken into account to recreate the palynofloral and tectonic history for a particular area.
radiolarians
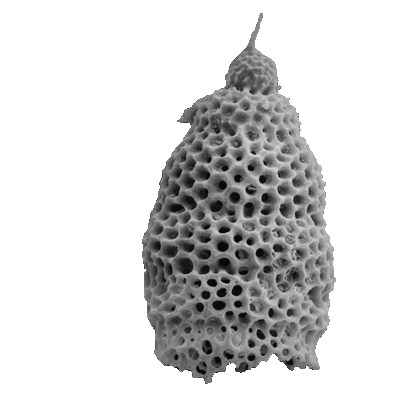
Radiolaria are holoplanktonic protozoa and form part of the zooplankton. They non-motile (except when flagella-bearing reproductive swarmers produced) but contain buoyancy enhancing structures; may be solitary or colonial. formally belong to phyllum protista, subphylum sarcodina, class actinopoda, subclass radiolaria. Sister acantharia have skeletons composed strontium sulphate which is easily dissolved in seawater not preserved fossil record. Within there two important super-orders. tripylea includes phaedaria hollow silica bars joined by organic material, commonly preserved, polycystina pure opal therefore more resistant dissolution hence divided into suborders spumellaria nassellaria. Wholly marine, most relatively studied members formal it must remembered, however, that under saturated with respect degree preservation depends on robustness skeleton, depositional burial conditions diagenesis.
The name Radiolaria was first used by Meyer in the early 19th Century. Haeckel's book of 1862 is full of fabulous illustrations which are available online thanks to Hamburg University see http:www.biologie.uni-hamburg.de/b-online/radio/ The Challenger expedition of 1873-76 was a milestone in radiolaria, study not least because of the huge amount of material collected and the susequent large monograph by Haeckel. The 1970's with the advent of the Deep Sea Drilling Program saw another burst of research. During the 1950's W. Riedel showed how Radiolaria evolved rapidly and could therefore be utilised as biostratigraphic tools.
First recorded occurrences of Radiolaria are from the latest Pre-Cambrian, they are generally thought to have been restricted to shallow water habitats. By the Silurian deep water forms are believed to have evolved. All early Radiolaria are spumellarians, the first possible nassellarians appear in the Carboniferous and definite true nassellarians do not appear until the Triassic. During the late Palaeozoic Radiolaria show a gradual decline until the end of the Jurassic when there is a rapid diversification, this coincides with the diversification of the dinoflagellates which may have represented an increased source of food for the Radiolaria. It is thought that the evolution of diatoms in the Cretaceous may have had a significant effect on radiolarian evolution due to competition for silica (diatoms also use silica to build their skeleton); it is commonly accepted that radiolarian skeletons have become finer and less robust from this time.
Extant radiolaria are classified using features of both the preservable skeleton and the soft parts, which makes the classificaiton of fossil forms extremely difficult. Most workers in this field today use classification schemes based on Nigrini and Moore's and Nigrini and Lombari's works on modern and Miocene radiolarians. A major problem with radiolarian classification is that separate classifications have been established for the Palaeozoic, Mesozoic and Cenozoic, and little has been done to integrate them. The two suborders, the spumellarians and the nassellarians are subdivided into informal groups which equate to family level.
Radiolarian assemblages often contain 200-400 species so they can potentially be very useful biostratigraphic and palaeoenvironmental tools. They have an unusually long geological range, from latest Pre-Cambrian to Recent. Because Radiolaria have a skeleton composed of silica and have an extremely long geological range they have become useful in the study of sediments which lack calcareous fossils, either because of deposition below the CCD (Carbonate Compensation Depth) or because the strata being examined are too old. Cherts and particularly nodules within chert bands are often good sources for Radiolaria. Ophiolites and accretionary terrains often include chert bands and Radiolaria may be the only palaeontological aid available in these situations and as such have proved invaluable in the study of these geological settings.
Despite being single-celled protozoans Radiolaria are quite complex, sophisticated organisms. The body is divided into a central capsule which contains the endoplasm and nucleus (or nucleii) and the extracapsulum which contains peripheral cytoplasm composed of a frothy bubble-like envelope of alveoli and a corona of ray-like axopodia and rhizopodia. They feed on other zooplankton, phytoplankton and detritus using their axopodia and rhizopodia in a similar fashion to foraminifera, except that Radiolaria seldom possess pseudopodia and their rhizopodia are not as branching or anastomosing as in foraminifera. Symbiotic algae (including dinoflagellates) often occur in the extracapsulum. The central capsulum is separated from the extracapsulum by the central capsular wall, cytoplasmic strands called fusules link the central capsulum and extracapsulum via pores in this wall. Fusules are unique to Radiolaria and their close relatives the Acantharia. Because Radiolaria are heterotrophic they are not limited to the photic zone and have been found at water depths as great as 4000m. However, because many living Radiolaria contain symbiotic photosynthesising algae they must spend at least daylight hours within the photic zone. Skeletal elements of radiolaria are covered with a layer of cytoplasm which is rapidly withdrawn if the organism is disturbed. It is suggested new skeletal material is formed within this sheath (called the cytokalyamma) and that it acts somehow like a dynamic mould.
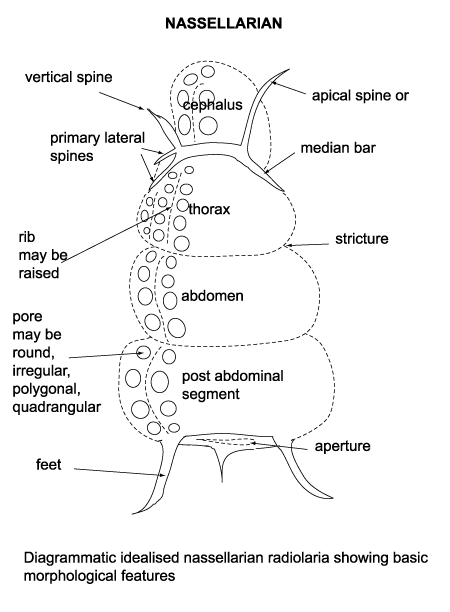
Simple asexual fission of radiolarian cells has been observed. Sexual reproduction has not been confirmed but is assumed to occur; possible gametogenesis has been observed in the form of "swarmers" being expelled from swellings in the cell. Swarmers are formed from the central capsule after the ectoplasm has been discarded. The central capsule sinks through the water column to depths hundreds of meters greater than the normal habitat and swells, eventually rupturing and releasing the flagellated cells. Recombination of these cells, which are assumed to be haploid, to produce diploid "adults" has not been observed however and is only inferred to occur. Comparisons of standing crops within the water column and sediment trap samples have ascertained that the average life span of radiolarians is about two weeks, ranging from a few days to a few weeks.
Radiolaria are often found in standard micropalaeontological preparations (i.e. those aimed at recovering foraminifera). However for the best results samples are washed using a weak (10%) concentration of hydroflouric acid. It is also possible to differentially etch Radiolaria from cherts using hydrofluoric acid. This is extremely dangerous and must only be carried out in a fume cupboard with full protective clothing and as such should be left to trained individuals only.
Radiolaria are often smaller than foraminifera but may be veiwed using the same techniques as those described for foraminifera, and they can be picked and mounted in the same way. They can also be prepared in strew mounts on glass slides.
The following images are of a representative selection of Radiolaria aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and, if possible, a species name followed by its age range, the site location from which the sample was obtained and its size in microns. LM (Light Microscope) SEM (Scanning Electron Microscope). Typical and selected marker species are illustrated from each main period of the geological column in which Radiolaria occur. The images are divided into Cenozoic, Mesozoic and Palaeozoic forms, click on a link below or scroll down to each section. Click on an image to view a larger version.
| Cenozoic |
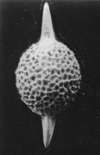 |
| Auxoprunum stauraxonium Haeckel |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| overall length 280 microns SEM |
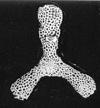 |
| Euchitonia furcata Ehrenberg |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| overall length 170 microns SEM |
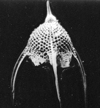 |
| Pterocanium praetaxum (Ehrenberg) |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| overall length 170 microns SEM |
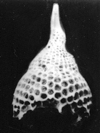 |
| Lamprocyclus maritalus Haeckel |
| ?Lower Oligocene-Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| overall length 180 microns SEM |
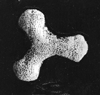 |
| Dictyocoryne truncatum (Ehrenberg) |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| overall length 90 microns SEM |
 |
| Spongaster tetras tetras Ehrenberg |
| Upper Pliocene-Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| overall length 180 microns SEM |
 |
| Actinomma sp. |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| 190 microns SEM |
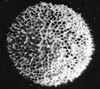 |
| Actinomma arcadophorum Haeckel |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| 180 microns SEM |
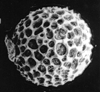 |
| Cenosphaera cristata Haeckel |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| 180 microns SEM |
 |
| Porodiscus sp. |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| 170 microns SEM |
 |
| Porodiscus sp. |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| 170 microns LM |
 |
| Pterocanium sp. |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| LM |
 |
| Botrystrobus aquilonarius (Bailey) |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| LM |
 |
| Pylospira octapyle Haeckel |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| LM |
 |
| Anthrocyrtidinium oriphense (Ehrenberg) |
| ?Recent |
| Owen Basin, Arabian Sea, N.W. Indian Ocean |
| LM |
 |
| Stylosphaera sp. |
| Rio Claro, Eastern Andalusia, Spain |
| 300 microns excluding spines SEM |
 |
| Stylochlamydium asteriscus Haeckel |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Stylochlamydium asteriscus Haeckel |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Cyrtocapsella cornuta Haeckel |
| Lower-Middle Miocene |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Dictyocoryne spp. Ehrenberg |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Stylosphaera sp. Ehrenberg |
| Rio Claro, Eastern Andalusia, Spain |
| 150 microns SEM |
 |
| Lamprocyclas maritalis Haeckel |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Lamprocyclas maritalis Haeckel |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Stichocorys delemontensis (Campbell and Clark) |
| Lower Miocene-Upper Miocene |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
 |
| Lychnocanoma elongata (Vinassa de Regny) |
| Lower Miocene |
| Rio Claro, Eastern Andalusia, Spain |
| SEM |
| Mesozoic |
 |
| Archaeospongoprunum cortinaensis (Pessagno) |
| ?Upper Cretaceous? |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Archaeospongoprunum cf. bipartum Pessagno |
| Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Halesium triacanthum (Squinabol) |
| Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Triactoma hexeris (O'Dogherty) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Paronaella grapevinensis (Pessagno) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
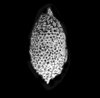 |
| Distylocapsa veneta (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Vitorfus minimus (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Acanthoicircus tympanum (O'Dogherty) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Thanarla venta (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Patellula cognata (O'Dogherty) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Alievium superbum (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Alievium superbum (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM (close up of pore frames) |
 |
| Ultranapora cretacea (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Dictyomitra montisserei (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
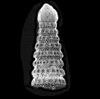 |
| Pseudodictyomitra pseudomarcrocephala (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Crucella cachensis Pessagno |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
 |
| Sciadiocapsa radiata (Squinabol) |
| ?Upper Cretaceous |
| Hacho de Montejaque, Penibetic, Southern Spain |
| SEM |
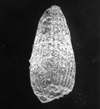 |
| Archaeodictyomitra sp. |
| Jurassic-Cretaceous |
| Theokafta, Epidavros, Greece |
| 140 microns SEM |
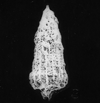 |
| Hsuum maxwelli Pessagno |
| ?Jurassic-Cretaceous? |
| Theokafta, Epidavros, Greece |
| 200 microns SEM |
 |
| Unuma echinatus Ichikawa and Yao |
| Aalanian-Bathonian (Jurassic) |
| Theokafta, Epidavros, Greece |
| 180 microns SEM |
 |
| Protunuma fusiformis Ichikawa and Yao |
| Bathonian-Tithonian (Jurassic) |
| Theokafta, Epidavros, Greece |
| 190 microns SEM |
 |
| Zamoidellum ovum Dumitrica |
| Oxfordian-Tithonian (Jurassic) |
| Theokafta, Epidavros, Greece |
| 110 microns SEM |
 |
| Tricolocapsa plicarum Yao |
| Bajocian-Bathonian (Jurassic) |
| Theokafta, Epidavros, Greece |
| 130 microns SEM |
 |
| Parvicingula dhimenaensis Baumgartner |
| Bajocian-Tithonian (Jurassic) |
| Theokafta, Epidavros, Greece |
| 255 microns SEM |